Coordination Compounds : Notes and Study Materials -pdf
Notes and Study Materials
- Concepts of Coordination Compounds
- Master File Coordination Compounds
- NCERT Solutions for – Coordination Compounds
- NCERT Exemplar Solutions for – Coordination Compounds
- Mind Map of Coordination Compounds
- Past Many 12th Board Years of Coordination Compounds
Examples and Exercise
CBSE Class 12th Chemistry Notes: Coordination Compounds
Get important notes on CBSE Class 12th Chemistry, Chapter 9: Coordination Compounds. These notes are very useful for quick revision before the exams.
CBSE Class 12 Chemistry: Chapter 9 – Coordination Compounds. These notes will give you a quick glance of the chapter. These notes are prepared strictly based on the latest CBSE syllabus for CBSE Class 12th Chemistry.
The main topics covered in these quick notes are:
• Definition of
o Coordination compounds o Double salt
o Central atom or ion
• Ligand and its types
• Chelate, Chelating ligands and Chelation
• Coordination number and Oxidation number
• Nomenclature of coordination compounds
• Isomerism in coordination compounds and its types
• Werner’s theory of coordination compounds
• Valence bond theory (VBT)
o Principles involved
o Limitations
• Crystal field splitting theory (CFT)
o Crystal field splitting in octahedral complexes
o Crystal field splitting in tetrahedral complexes
o Stability of coordination compounds
o Colour in Coordination Compounds
o Limitations of crystal field theory
• Metal carbonyls
o Structure of some important metal carbonyls
o Bonding in metal carbonyls
o Properties of metal carbonyls
• Applications of coordination compounds
Double salt
When two salts in stoichiometric ratio are crystallised together from their saturated solution they are called double salts. They are stable in solid state but dissociate into constituent ions when dissolved in water.
For example: FeSO4. (NH4) 2SO4.6H2O (Mohr’s salt)
Central atom or ion
The atom or ion to which a fixed number of ions or groups are bound in a definite geometrical arrangement around it, is called the central atom or ion.
For example: In K4[Fe(CN)6], Fe2 is the central metal ion.
Ligands
The atoms or groups which are attached directly to central atoms are called ligands. Ligands are Lewis bases which donates electron pair and forms coordinate bonds with the metal atom.
For example: H2O, CO, NO2‒, etc.
A ligand may be neutral, positively or negatively charged.
Types of ligands
On the basis of charge present, ligands may be categorized into the following three groups:
Anionic ligands: CN‒ (cyanide), NO2‒ (nitrito-N), NO3‒ (nitrito), X‒ (halido), etc.
Cationic ligands: NO2+ (nitrosonium), NO+ (nitronium), N2F5+ (Hydrazenium), etc.
Neutral ligands: NH3 (ammine), H2O (aqua), CO (carbonyl), etc.
On the basis of number of coordinating atoms present ligands may be classified as follows:
Monodentate: It is the ligand which has only one coordinating atom.
For example: X‒ (halido), NH3 (ammine), H2O (aqua), CN‒ (Cynaido) .
Diddentate: It is the ligand which has two coordinating sites available.
For example: H2NCH2CH2NH2 (ethane-1, 2-diamine) and C2O42‒ (oxalate).
Polydentate: Ligand containing several donor sites are called polydentate.
For example: N(CH2CH2NH2)3 (Nitrilotriethylamine, a tetradentate ligand )
EDTA (Ethylene Diamine tetraetato ion): It is a hexadentate ligand.
Ambidentate ligand: This is the ligand which can ligate through two different atoms present in it.
For examples: NO2− ion can coordinate either through nitrogen or through oxygen to a central metal atom/ion.
Chelate, Chelating ligands and Chelation
Di- and ploydentqte ligands results in the formation of cyclic structure around the central metal atom. Such cyclic metal complex is called chelate and the ligands which gives chelates are called chelating ligands, and the process is known as chelation.
Denticity
Denticity is defined as the number of coordinating atoms present per ligand.
Coordination number
It is defined as the number of coordinate bonds formed by central metal atom, with the ligands. For example: In K4[Fe(CN)6], Fe has coordination number 6.
Oxidation number
The oxidation number of the central atom is defined as the charge left on a given atom when all other atom in a complex are removed as ions. The oxidation number is indicated by Roman numerals.
Nomenclature of coordination compounds
Step to name a complex compound are:
Cations are always named before the anions.
The ligands are then listed in alphabetical order.
In case of polydentate ligands, ligands are named alphabetically using a prefix di, tri, tetra, penta etc, to indicate the number of ligands of that type present.
Then the name the metal atom is written followed by its oxidation state in Roman numerals.
Finallythe anion is named.
For example: [Cu(NH3)4]SO4 Tetra ammine copper (II) sulphate
If the ligands itself include di, tri etc. then we use bis – (for two), tris (for three) as prefix. For example:
[PtCl2(en)2]2+ Dichloridobis (1, 2 – ethanediamine) platinum (IV) ion.
[Co(en)3]3+ Tris(ethanediamine)cobalt (III) ion.
If the complex ion is an anion, the name of the metal ends with the suffix – ate.
For example: [Cr(C2O4)3]3‒ Trioxalatochromate (III) ion.
Isomerism in coordination compounds
Isomers are two or more compounds that have the same chemical formula but a different arrangement of atoms.
Types of Isomerism
There are two types of isomerism:
(a) Stereoisomerism
(i) Geometrical isomerism (ii) Optical isomerism
(b) Structural isomerism
(i) Linkage isomerism (ii) Coordination isomerism
(iii) Ionisation isomerism (iv) Solvate isomerism Structural isomerism
Stereoisomerism
It arises due to different arrangement of atoms or groups in space. Two different types of stereoisomerism are described as follows:
(i) Geometrical isomerisms:
It arises in heteroleptic complexes due to different possible geometrical arrangements of ligands. In square planar complex of formula [MX2L2] (where X and L are unidentate), the two ligands X may be arranged adjacent to each other in a cis isomer, or opposite to each other in a trans isomer.
For example: Geometrical isomers of Pt(NH3)2Cl2) are shown below:
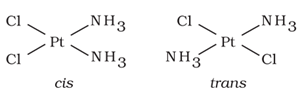
Similarly in octahedral complexes of formula [MX2L4], two ligands X may be oriented cis or trans to each other.
For example: Geometrical isomers of [Co(NH3)4Cl2] are shown below:
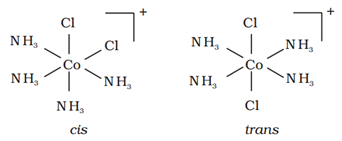
(ii) Optical isomerism:
It arises due to the presence of non-super imposable mirror images. The non-superimposable mirror images are called enantiomers.
The enantiomers reacts differently with the plane polarised light.
The enantiomer which rotate the plane polarised light in a clockwise direction is called dextrorotatry (d) or (+) and the enantiomer which rotate the plane polarised light in anticlockwise direction is called lavorotatory (l) or (‒).
For example: Optical isomers of [Co(en)3 ] 3+ are shown below:
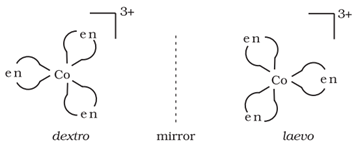
Structural isomerism
It arises due to the difference in structures of coordination compounds. Four different types of structural isomerism are described as follows:
Ionisation isomerism: These are the isomers having the same molecular formula but gives different ions in solution.
For example: [Cr(NH3)Br]SO4 and [Cr(NH3)(SO4)]Br are ionization isomers.
Linkage isomerism: Isomers having the same molecular formula but different linking atoms are named as linkage isomers. This arises due to the presence of ambident ligands.
For example:[CO(NH3)5(NO2)]2+ and [CO(NH3)5 (ONO)]2+ are linkage isomers.
Coordination isomerism: This type of isomerism is possible when cation and anion both are complex and isomerism arises due to complete exchange of coordination sphere.
For example: [Pt (NH3)4] [Ni (CN)4] and [Ni (NH3)4] [Pt (CN)4] are coordination isomers.
Hydration isomerism: Isomers having the same molecular formula but different water molecules of hydration are known as hydration isomers of each other.
For example: [Cr (H2O)5Cl]Cl2.H2O and [Cr(H2O)4Cl2]Cl.2H2O are hydration isomers.
Werner’s theory of coordination compounds
According to Werner’s theory of coordination compounds, there are two types of valencies in coordination compounds:
o Primary valencies: These are ionizable valencies, satisfied by anions and determines the charge on the complex ions.
o Secondary valencies: These are non-ionisable valencies, satisfied by ligands and determines the coordination number of the metal atom.
Valence Bond Theory
Valence bond theory states that the metal atom or ion under the influence of ligands can use its (n-1)d, ns, np or ns, np, nd orbitals for hybridisation to yield a set of equivalent orbitals of definite geometry such as octahedral, tetrahedral, square planar and so on. These hybridised orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding.
Following table shows the combination of different number of orbitals to give different types of hybridization:
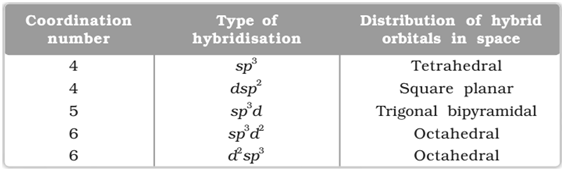
Thus the basic principles involved in the valence bond theory are:
• Hybridisation of orbitals
• Bonding between the ligands and the metal atoms/ion
• Relation between the observed magnetic moment and the bond type
Limitation of the VB theory
• It does not tell anything about the spectral properties of the complexes.
• It does not give quantitative interpretation of magnetic data.
• It does not distinguish between strong and weak ligands.
• It does not explain the colour exhibited by coordination compounds.
• It does not give a quantitative interpretation of the thermodynamic or kinetic stabilities of coordination compounds.
Magnetic properties of coordination compounds
A coordination compound is paramagnetic in nature if it has unpaired electrons and diamagnetic if all the electrons in the coordination compound are paired.
Crystal Field Theory
In crystal field theory (CFT), ligands are considered as point charges and the interaction between the ligands and the metal ion is purely electrostatic in nature. The five d-orbitals in an isolated gaseous metal atom/ion have same energy, i.e., they are degenerate. The degeneracy is lost in the presence of the ligand field.
The five d-orbitals are classified as:
Three d-orbitals, dxy, dyz and dzx that are oriented in between the coordinate axes and are called t2g –orbitals.
Two d-orbitals, dx2 ‒ y2 and dz2 that are oriented along the x – y axes and are called eg – orbitals.
Factors affecting the splitting of d-orbitals
• Nature of the ligand
• Nature of the metal ions
• Geometry of complex whether it is octahedral or tetrahedral
• Oxidation state of the metal ion
Crystal field splitting in octahedral complexes
Here energy of eg set of orbitals > energy of t2g set of orbitals.
Ligands for which energy separation, Δo < P (the pairing energy, i.e., energy required for
electron pairing in a single orbital) form a high spin complex.
Ligands for which energy separation, Δo > P, form low spin complex.
Crystal field splitting in tetrahedral complexes
Here energy of t2g set of orbitals > Energy of eg set of orbitals.
In such complexes d-orbital splitting is inverted and is smaller as compared to the octahedral field splitting.
No pairing of electrons is possible due to the lowest splitting energies which leads to high spin complexes.
Stability of coordination compounds
The stability of the coordination compound depends on
Nature of the ligand
Chelating ligands form strong and more stable complexes than the monodentate ligands. The π– bond ligands forms more stable complexes than the σ– bonded complex.
Nature of the metal atom/ion
Small, highly charged metal ions form more stable complexes than large size, lowly charged metal ion.
Colour in Coordination Compounds
The crystal field theory attributes the colour of the coordination compounds to d-d transition of the electron, i.e., the transiton of electron from t2g level to the higher eg level which accompanies the absorption of light in visible spectrum.
In the absence of ligands, crystal field splitting does not occur and hence the substance is colourless.
Limitations of crystal field theory
It does not take into account the partly covalent character of bonding between the ligand and the central atom.
It is also unable to explain the relative strengths of ligands e.g., it does not explain why H2O is stronger ligand than OH−.
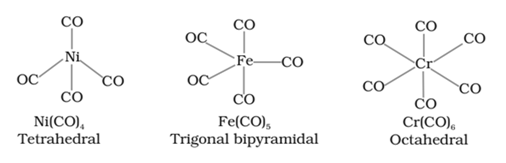
Metal carbonyls
The homoleptic complexes in which carbon monoxide (CO) acts as the ligand are called metal carbonyls.
For example: Ni(CO)4
Structure of some important metal carbonyls are:
Bonding in metal carbonyls
• The metal-carbon bond in metal carbonyls possess both s and p character. CO as a ligand binds itself to metal atoms through the carbon atom to form the metal-carbon (M-C) bond. It is a weak donor.
• The M–C σ bond is formed by the donation of lone pair of electrons on the carbonyl carbon into a vacant orbital of the metal.
• The M–C π bond is formed by the donation of a pair of electrons from a filled d orbital of metal into the vacant antibonding π* orbital of carbon monoxide. This characteristic property of back bonding which stabilises the metalligand interaction is termed as synergic effect.
Properties of metal carbonyls
• They are generally solids at room temperature and pressure except Ni(CO)4 and Fe (CO)5.
• Mononuclear carbonyls are volatile and toxic.
• Mononuclear carbonyls are either colourless or light coloured
Applications of coordination compounds
• EDTA is used in the estimation of Ca2+ and Mg2+ in hardwater. The Ca2+ and Mg2+ ions form stable complexes with EDTA.
• Metals can be purified by the formation and subsequent decomposition of their coordination compounds. For example, impure nickel is converted to [Ni(CO)4], which is decomposed to yield pure nickel.
• In analytical chemistry [Ni(DMG)2]2+ complex is used in the detection of Ni in chocolates.
• In medicine, cisplatin, a cis isomer of [Pt(Cl)2(NH3)2] is used in the treatment of cancer.
• Solutions of the complexes like [Ag(CN)2]– and [Au(CN)2]– can be used for the smooth and even electroplating of metals by gold or silver.
• Chlorophyll, a pigment responsible for photosynthesis, is a coordination compound of magnesium. Also haemoglobin, the red pigment of blood which acts as oxygen carrier is a coordination compound of iron.
Coordination Compounds Class 12 Chemistry MCQs
1. The sum of coordination number of oxidation number of the metal M in the complex [M(en)2 C204] Cl are
(a) 7
(b) 8
(c) 9
(d) 6
Answer/Explanation
Answer: c
Explaination: (c) 9 = 6 + 3
2. Which of the following will not give test for Cl– with AgNO3(aq) at 25°C?
(a) COCl3.5NH3
(b) COCl3.6NH3
(c) COCl3.3NH3
(d) COCl3.4NH3
Answer/Explanation
Answer: c
Explaination:
(c) [Co(NH3)3 Cl3) does not have counter ions, so will not react with AgNO3(aq)
3. Which of these statements about [Co(CN)6]3- is true?
(a) It has 4 unpaired electron, high spin
(b) No unpaired electron, high spin
(c) No unpaired electron, low spin
(d) 4 unpaired electron, low spin
Answer/Explanation
Answer: c
Explaination:
(c) [CO(CN)6]3- has d²sp3 hybridisation, no unpaired electron, low spin.
4. The correct order of the stoichiometries of AgCl formed when AgNO3 in excess is treated with complexes: COCl3.6NH3, C0Cl3.5NH3, C0Cl3.4NH3 respectively is
(a) 3AgCl, lAgCl, 2AgCl
(b) 3AgCl, 2AgCl, 1AgCl
(c) 2AgCl, 3AgCl, 2AgCl
(d) lAgCl, 3AgCl, 2AgCl
Answer/Explanation
Answer: b
Explaination:
(b) [Co(NH3)6Cl3], [CO(NH3)5Cl] Cl2,
[CO(NH3)4Cl2], will form 3AgCl, 2AgCl and lAgCl respectively.
5. Correct increasing order of wavelength of absorption in visible region for complex of Co3+ is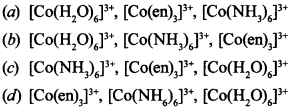
Answer/Explanation
Answer: d
Explaination:
(d) ∵ en is bidentate ligand will form stable complex will absorb highest energy but lowest wavelength, [Co(H2O)6]3+ will absorb lowest energy, highest wavelength.
6. Pick out the correct statement with respect to [Mn(CN)J2-
(a) It is sp²d² hybridised, tetrahedral
(b) It is d²sp3 hybridised, octahedral
(c) It is dsp² hybridised, square planar
(d) It is sp3d² hybridised octahedral
Answer/Explanation
Answer: b
Explaination: (b) d²sp3, inner orbital complex.
7. Facial and meridional isomerism will be shown by
(a) [Co(NH3)3Cl3]
(b) [Co(NH3)4Cl2] Cl
(c) [Co(en)3] Cl3
(d) [Co(NH3)5Cl] Cl2
Answer/Explanation
Answer: a
Explaination: (a) will show fac-mer isomerism
8. Which one has highest molar conductivity?
(a) [Pt(NH3)2 Cl2]
(b) [CO(NH3)4 Cl2] Cl
(c) K4[Fe(CN)6]
(d) [Cr(H2O)6] Cl3
Answer/Explanation
Answer: c
Explaination: (c) will have highest molar conductivity.
9. Which one will show optical isomerism?
(a) [Co(NH3)3Cl3]
(b) cis-[Co(en)2 Cl2] Cl
(c) trans-[Co(en)2 Cl2] Cl
(d) [Co(NH3)4 Cl2] Cl
Answer/Explanation
Answer: b
Explaination:
(b) will show optical isomerism because it does not have symmetry and polydentate ligand.
10. The pair having the same magnetic moment is (At No. Cr = 24, Mn = 25, Fe = 26, Co = 27)
(а) [Cr(H2O)6]2+ and [CoCl4]2-
(b) [Cr(H2O)6]2+ and [Fe (H2O)6]2+
(c) [Mn(H2O)6]2+ and[Cr(H2O)6]2+
(d) [COCl4]2- and [Fe(H2O)6]2+
Answer/Explanation
Answer: b
Explaination:
(b) [Cr(H2O)6]2+ has 4s°3d4 electronic configuration, has four unpaired electrons. [Fe(H2O)6]2+ has 4s°3d6 has four unpaired electrons.
11. On treatment of 100 mL of 0.1 MCOCl3.6H2O with excess of AgNO3, 1.2 × 1022 ions are precipitated. The complex is
(a) [Co(H2O)4Cl2]Cl.2H2O
(b) [Co(H2O)3Cl3]3H2O
(c) [Co(H2O)6] Cl3
(d) [Co(H2O)5 Cl] Cl2.H2O
Answer/Explanation
Answer: d
Explaination:
(d) [Co(H2O)5 Cl] Cl2.H2O + 2AgNO3 (aq)
100 × 0.1 = 10 millimoles
→ 2AgCl↓ + [Co(H2O)5Cl](NO3)2.H2O 20 millimoles of AgCl
= 20 × 10-3 × 6 × 1023
= 120 × 1020 = 1.2 × 1022
∴ 1.2 × 1022 ions are precipitated.
12. Among the ligands NH3, en, CN and CO, the correct order of field strength is
(a) NH3 < en < CN– < CO
(b) CN– < NH3 < CO < en
(c) en < CN–< NH3 < CO
(d) CO < NH3 < en < CN–
Answer/Explanation
Answer: a
Explaination: (a) is correct order.
13. Which cf the following complexes formed by Cu2+ ions is most stable? [NCERT Exemplar]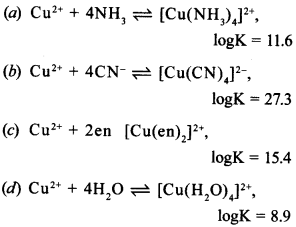
Answer/Explanation
Answer: b
Explaination:
(b) is most stable, higher the value of ‘K’, more will be log K, therefore, more stable will be complex.
14. The stabilisation of coordination compounds due to chelation is called the chelate effect. Which of the following is the most stable complex species? [NCERT Exemplar]
(a) [Fe(CO)5]
(b) [Fe(CN)6]3-
(c) [Fe(C2O4)3]3-
(d) [Fe(H2O)6]3+
Answer/Explanation
Answer: c
Explaination:
(c) is most stable because C2O4– is bidentate ligand.
15. Indicate the complex ion which shows geometrical isomerism.
(a) [Cr(H2O)4Cl2]+
(b) [Pt(NH3)3 Cl]
(c) [Co(NH3)6]3+
(d) [Co(CN)5(NC)]3-
Answer/Explanation
Answer: a
Explaination: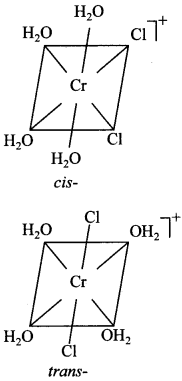
16. The CFSE for octahedral [CoCl6]4- is 18,000 cm-1. The CFSE for tetrahedral [CoCl4]2- will be [NCERT Exemplar]
(a) 18,000 cm-1
(b) 16,000 cm-1
(c) 8,000 cm-1
(d) 20,000 cm-1
Answer/Explanation
Answer: c
Explaination:
(c) ∆t = \(\frac{4}{9}\) ∆0 = \(\frac{4}{9}\) × 18000 = 8000
17. Due to the presence of ambidentate ligands coordination compounds show isomerism. Palladium complexes of the type [Pd(C6H5)2(SCN)2] and [Pd(C6H5)2(NCS)2] are [NCERT Exemplar]
(a) linkage isomers
(b) coordination isomers
(c) ionisation isomers
(d) geometrical isomers
Answer/Explanation
Answer: a
Explaination:
(a) SCN is ambidentate ligand, therefore, it shows linkage isomerism.
18. The compounds [Co(SO4)(NH3)5]Br and [CO(SO4)(NH3)5]Cl represent [NCERT Exemplar]
(a) linkage isomerism
(b) ionisation isomerism
(c) coordination isomerism
(d) no isomerism
Answer/Explanation
Answer: b
Explaination:
(b) It shows ionisation isomerism v counter ions can act as ligand.
19. A chelating agent has two or more than two donor atoms to bind to a single metal ion. Which of the following is not a chelating agent? [NCERT Exemplar]
(a) thiosulphato
(b) oxalato
(c) glycinato
(d) ethane-1, 2-diamine
Answer/Explanation
Answer: a
Explaination:
(a) S2O32- (thiosulphato) ion is unidentate ligand and is not a chelating agent.
20. The solution of the complex [Cu(NH3)4] SO4 in water will
(a) give the tests of Cu2+ ion
(b) give the tests of NH3
(c) give the tests of SO42- ions
(d) not give the tests of any of the above
Answer
Answer: c
21. IUPAC name of [Pt(NH3)3 Br (NO2) Cl] Cl isw
(a) triamminechlorodibromidoplatinum (IV) chloride
(b) triamminechloridobromidonitrochloride- platinum (IV) chloride
(c) triamminebromidochloridonitroplatinum (IV) chloride
(d) triamminenitrochlorobromoplatinum (IV) chloride
Answer
Answer: c
23. Trunbull’s blue is
(a) Ferricyanide
(b) Ferrous ferricyanide
(c) Ferrous cyanide
(d) Fe3[Fe(CN)6]4
Answer
Answer: b
23. Primary and secondary valency of Pt in [Pt(en)2Cl2] are
(a) 4, 4
(b) 4, 6
(c) 6, 4
(d) 2, 6
Answer
Answer: d
24. The complex ions [Co(NH3)5(NO2)]2+ and [Co(NH3)5 (ONO)]2+ are called
(a) Ionization isomers
(b) Linkage isomers
(c) Co-ordination isomers
(d) Geometrical isomers
Answer
Answer: b
25. Which of the following has square planar structure?
(a) [NiCl4]2-
(b) [Ni(CO)4]
(c) [Ni(CN)4]2-
(d) None of these
Answer
Answer: c
26. Which of the following has magnesium?
(a) Chlorophll
(b) Haemocyanin
(c) Carbonic anhydrate
(d) Vitamin B12
Answer
Answer: a
27. Mohr’s salt is
(a) Fe2(SO4) 3 . (NH4)2SO4 . 6H2O
(b) FeSO4 . (NH4)2 . SO4 . 6H2O
(c) MgSO4 . 7H2O
(d) FeSO4 . 7H2O
Answer
Answer: b
28. Which of the following shall form an octahedral complex?
(a) d4 (low spin)
(b) d8 (high spin)
(c) d6 (low spin)
(d) All of these
Answer
Answer: b
29. EDTA is used for the estimation of
(a) Na+ and K+ ions
(b) Cl– and Br– ions
(c) Cu2+ and Cs+ ions
(d) Ca2+ and Mg2+ ions
Answer
Answer: d
30. The name of complex [Fe(CN)6]3- is
(a) Tricyanido ferrate (III) ion
(b) Hexacyanido ferrate (III) ion
(c) Hexacyanido iron (III)
(d) Hexacyanido ferrate (II) ion
Answer
Answer: b
Note: In the following questions two or more options may be correct. (Q.21 to Q.25)
31. Atomic number of Mn, Fe and Co are 25,26
and 27 respectively. Which of the following inner orbital octahedral complex ions are diamagnetic? [NCERT Exemplar]
(a) [CO(NH3)6]3+
(b) [Mn(CN)]3-
(c) [Fe(CN)6]4-
(d) [Fe(CN)6]3-
Answer/Explanation
Answer: a
Explaination:
(a) [Co(NH3)6]3+ and (c) [Fe(CN)6]4- are diamagnetic due to absence of unpaired electron.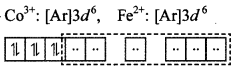
d²sp3 hybridisation.
32. Atomic number of Mn, Fe, Co and Ni i are 25, 26, 27 and 28 respectively. Which of the following outer orbital octahedral ’complexes have same number of unpaired electrons? [NCERT Exemplar]
(a) [MnCl6]3-
(b) [FeF6]3-
(c) [CoF6]3-
(d) [Ni(NH3)6]2+
Answer/Explanation
Answer:
Explaination:
(a) and (c).![]()
Both have same number of unpaired electrons.
33. Which of the following options are correct for [Fe(CN)6]3- complex? [NCERT Exemplar]
(a) d²sp3 hybridisation
(b) sp3cf hybridisation
(c) paramagnetic
(d) diamagnetic
Answer/Explanation
Answer:
Explaination:
(a) and (c) are d²sp3 and paramagnetic.
34. An aqueous pink solution of cobalt(II) chloride changes to deep blue on addition of excess of HC1. This is because _________ . [NCERT Exemplar]
(a) [Co(H2O)6]2+ is transformed into [CoCl6]4-
(b) [Co(H2O)6]2+ is transformed into [CoCl4]2-
(c) tetrahedral complexes have smaller crystal field splitting than octahedral complexes.
(d) tetrahedral complexes have larger crystal field splitting than octahedral complex.
Answer/Explanation
Answer:
Explaination:
(b) and (c) are correct statement ∆t = \(\frac{4}{9}\) ∆0
35. Identify the optically active compounds from the following: ]NCERT Exemplar]
(a) [Co(en)3]3+
(b) trans- [Co(en)2 Cl2]+
(c) cis- [Co(en)2 Cl]+
(d) [Cr (NH3)5Cl]
Answer/Explanation
Answer:
Explaination:
(a) and (c) are optically active compounds.
36. Math the complex ions given in Column I with the colours given in Column II and assign the correct code:
| Column I (Complex ion) | Column II (Colour) |
| (A) [Co(NH3)]3+ | (1) Violet |
| (B) [Ti(H2O)6]3+ | (2) Green |
| (C) [Ni(H2O)6F | (3) Pale blue |
| (D) (Ni (H2O)4(en)]2+(aq) | (4) Yellowish orange |
| (5) Blue |
Code: (i) A (1) B (2) C (4) D (5)
(ii) A (4) B (3) C (2) D (1)
(iii) A (3) B (2) C (4) D (1)
(iv) A (4) B (1) C (2) D (3)
Answer/Explanation
Answer:
Explaination:
(ii) A (4) B (3) C (2) D (1)
37. Match the coordination compounds given in Column I with the central metal atoms given in Column II and assign the correct code: [NCERT Exemplar]
| Column I (Coordination compound) | Column II (Central metal atom) |
| (A) Chlorophyll | (1) rhodium |
| (B) Blood pigment | (2) cobalt |
| (C) Wilkinson catalyst | (3) calcium |
| (D) Vitamin B | (4) iron |
| (5) magnesium |
Code: (i) A (5) B (4) C (1) D (2)
(ii) A (3) B(4) C (5) D (1)
(iii) A (4) B (3) C (2) D (1)
(iv) A (3) B (4) C(l) D (2)
Answer/Explanation
Answer:
Explaination:
(i) A (5) B (4) C (1) D (2)
38. Match the i complex ions given in Column with the hybridisation and number of unpaired electrons given in Column II and assign the correct code: [NCERT Exemplar]
| Column I (Complex ion) | Column II (Hybridisation, number of unpaired electrons) |
| (A) [Cr(H2O)6]3+ | (1) dsp2, 1 |
| (B) [Co(CN4)]2- | (2) sp3d2, 5 |
| (C) [Ni(NH3)6]2+ | (3) d2sp3, 5 |
| (D) [MnF6]4- | (4) sp3, 4 |
| (5) sp3d2, 2 |
Code: (i) A (3) B (1) C(5) D (2)
(ii) A (4) B (3) C (2) D (1)
(iii) A (3) B (2) C (4) D (1)
(iv) A (4) B (1) C (2) D (3)
Answer/Explanation
Answer:
Explaination:
(i) A (3) B (1) C (5) D (2)
39. In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are hue, reason is correct explanation of assertion.
(b) Assertion and reason both are true but reason is not the correct explanation of assertion.
(c) Assertion is true, reason is false.
Assertion: Toxic metal ions are removed by the chelating ligands. [NCERT Exemplar]
Reason: Chelate complexes tend to be more stable.
Answer/Explanation
Answer: a
Explaination:
(a) Assertion and reason both are true reason is correct explanation of assertion.
40. If ∆o > P for d4, electronic configuration in terms of CFT is _________ .
Answer/Explanation
Answer:
Explaination:
\(t_{2 \mathrm{g}}^{4} e g^{0}\)
41. If ∆o < P for d4, electronic configuration interms of CFT is _________ .
Answer/Explanation
Answer:
Explaination:
\(t_{2 \mathrm{g}}^{4} e g^{2}\)
42.![]()
are used for electroplating Ag and Au respectively. [True/False]
Answer/Explanation
Answer:
Explaination: True.
43. Ca2+ and Mg2+ are removed and estimated by using EDTA from hard water. [True/False]
Answer/Explanation
Answer:
Explaination: True.
44. Ni2+ forms scarlet red complex with dimethyl glyoxime. [True/False]
Answer/Explanation
Answer:
Explaination: True.
45. How many moles of AgCl will be precipitated when an excess of AgNO3 is added to a molar solution of [CrCl(H2O)5]Cl2? [Uttarakhand 2019]
Answer/Explanation
Answer:
Explaination:
2 moles of AgCl will be precipitated. [CrCl(H2O)5]Cl2 + 2AgNO3(aq) → [CrCl(H2O)5](NO3)2 + 2AgCl(s) (white ppt.)
46. Differentiate between a double salt and a complex. [Uttarakhand 2019]
Answer/Explanation
Answer:
Explaination:
A double salt dissociates into simple ions completely when dissolved in water such as camallite (KCl.MgCl2.6FI2O) while a complex ion such as [Fe(CN)6]4- does not dissociate into Fe2+.
47. What is the colour of [Co(NH3)6]3+ 3Cl?
Answer/Explanation
Answer:
Explaination: Yellow.
48. Give one similarity and one dissimilarity between primary and secondary valences.
Answer/Explanation
Answer:
Explaination:
Similarity: Both are satisfied by negative ions. Secondary valences are also satisfied by neutral molecules.
Dissimilarity: Primary valences are normally ionisable while secondary valences are non- ionsable.
49. What is meant by double salt?
Answer/Explanation
Answer:
Explaination:
Double Salt: When two salts in equimolar ratio are crystallised together from their saturated solution they are called double salts, e.g. FeSO4 .(NH4)2SO4.6H2O (Mohr’s salt). Potash alum is K2SO4.Al2(SO4)3. 24H2O.
50. Define coordination number.
Answer/Explanation
Answer:
Explaination:
Coordination number is defined as the number of donor atoms which form coordinate bonds with a central metal ion in complex compound or ion.
51. What is the coordination number of a central ion in octahedral complex? Why does NH3 form complex but NH4+ ion does not?
Answer/Explanation
Answer:
Explaination:
The coordination number of a central ion in octahedral complex is 6. NH3 acts as ligand because in NH3, nitrogen has lone pair of electron, whereas NH4+ does not have lone pair of electron and secondly, it is positively charged, therefore, it will be repelled by central metal ion.
52. What is the coordination number of Fe in [Fe(EDTA)]–?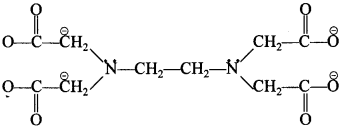
Answer/Explanation
Answer:
Explaination:
The coordination number of Fe is 6. It is because EDTA is hexadentate ligand, it can form six coordinate bonds on shown below. Two bonds are formed by two ‘N’ atoms having lone pair and four bonds are formed by four oxygen atoms having negative charge. f . ?
53. Write IUPAC name of K3[Fe(C2O4)3]. [Delhi 2013(C)]
Answer/Explanation
Answer:
Explaination: Potassium trioxalatoferrate(III).
54. Name the coordination compound: K3[CrF6]. [Foreign 2011]
Answer/Explanation
Answer:
Explaination: Potassium hexafluorido chromate(III)
55. Write the IUPAC name of [Pt(NH3)4Cl2]Cl2. [AI 2011(C)]
Answer/Explanation
Answer:
Explaination: Tetraaminedichloridoplatinum(IV) chloride
56. Write the IUPAC name of [Pt(NH3)3(NO)Cl2] Br2. [Delhi 2011(C)]
Answer/Explanation
Answer:
Explaination: Triamminedichloridonitrosyl platinum(IV) bromide.
57. Write the IUPAC name of [CO(CN)2(NH3)4]Cl. [Delhi 2011(C)]
Answer/Explanation
Answer:
Explaination: Tetraamminedicyanocobalt(III) chloride.
58. Write the IUPAC name of [PtCl(NH2CH3)(NH3)2]Cl. [Delhi 2011(C)]
Answer/Explanation
Answer:
Explaination: Diamminechlorido methanamine platinum(II) chloride.
59. Write the IUPAC name of [Cr(NH3)6] [Co(CN)6]. [AI 2011(C)]
Answer/Explanation
Answer:
Explaination: Hexaamminechromium(III) hexacyanoco- baltate(III).
60. Write IUPAC name and identify the ligands in complex ion [Co(en)2Cl(ONO)]+.
Answer/Explanation
Answer:
Explaination:
Ethane-1, 2-diamine (en), chlorido (Cl), nitrito—0 (ONO) are ligands. IUPAC name is chloridobis (Ethane-l,2-diamine)nitrito—0— cobalt(III).
61. Write the chemical formula for the complex compound: Sodium (ethylenediaminetetraacetate) chromate(II).
Answer/Explanation
Answer:
Explaination: Na4[Cr(EDTA)]
62. Which type of isomerism is shown by [Co(NH3)5ONO]2+ and [Co(NH3)5NO2]2+?
Answer/Explanation
Answer:
Explaination: Linkage isomerism.
63. What type of isomerism is exhibited by the complex [CO(NH3)5NO2]2+? [Foreign 2014]
Answer/Explanation
Answer:
Explaination: Linkage isomerism.
64. What type of isomerism is shown by the following complex: [Co(NH3)6][Cr(CN)6] [Delhi 2017; Foreign 2014]
Answer/Explanation
Answer:
Explaination: Coordination isomerism.
65. Write coordination isomer of [CO(NH3)6] [Cr(C2O4)3].
Answer/Explanation
Answer:
Explaination:
[Co(NH3)6][Cr(C2O4)3] has coordination isomer [Cr(NH3)6][Co(C2O4)3].
66. What type of isomerism is exhibited by the following complex: [CO(NH3)5SO4]Cl [Similar to CBSE 2018; Delhi 2017; Foreign 2014]
Answer/Explanation
Answer:
Explaination: Ionisation isomerism.
67. Give an example of ionisation isomerism.
Answer/Explanation
Answer:
Explaination:
[Co(NH3)5Br]SO4 and [Co(NH3)5SO4)]Br is the example of ionisation isomerism.
68. Give an example of solvate isomerism.
Answer/Explanation
Answer:
Explaination:
[Cr(H2O)6]Cl3 (violet) and its solvate isomer is [Cr(H2O0)5Cl] Cl2.H2O.
69. Give any two limitations of valence bond theory.
Answer/Explanation
Answer:
Explaination:
(i) It does not explain the colour indicated by coordination compounds.
(ii) It does not distinguish between strong and weak ligands.
70. Which has strong field strength SCN– or CN–?
Answer/Explanation
Answer:
Explaination: CN–. (According to spectrochemical series)
71. Why removal of water from [Ti(H2O)6] Cl3 on heating renders it colourless?
Answer/Explanation
Answer:
Explaination:
This is due to the fact that in the absence of ligand, crystal field splitting does not occur and hence the substance is colourless.
72. Give an example of homoleptic carbonyl.
Answer/Explanation
Answer:
Explaination: Tetracarbonylnickel(O).
73. What is meant by the instability constant of coordination compounds?
Answer/Explanation
Answer:
Explaination:
The reciprocal of the formation constant is called the instability constant of coordination compounds.
74. Name a coordination compound used in the treatment of lead poisoning.
Answer/Explanation
Answer:
Explaination: EDTA.
75. Write IUPAC name of the complex: [CoCl2(en)2]+.
Answer/Explanation
Answer:
Explaination: Dichloro bis (ethane 1,2-diamine) cobalt(II).