Solved CBSE Sample Papers for Class 10 Science Set 2
GENERAL INSTRUCTIONS
I. The Question Paper comprises of two sections, A and B. You are to attempt both the sections.
II. All questions are compulsory.
III. All questions of Section A and all questions of Section B are to be attempted separately.
IV. Question numbers I to 2 in Section A are one mark questions. These are to be answered in one word or in one sentence.
V. Question numbers 3 to 5 in Section A are two marks questions. These are to be answered in about 30 words each.
VI. Question numbers 6 to 15 in Section A are three marks questions. These are to be answered in about 50 words each.
VII. Question numbers 16 to 21 in Section A are five marks questions. These are to be answered in about 70 words each.
VIII. Question numbers 22 to 27 in Section B are questions based on practical skills and are five marks questions.
SECTION A
Question 1:
How is the concentration of hydronium (H3O+) ions affected when a solution of
an acid is diluted?
Answer:
When an acid solution is diluted with water then concentration of (H3O+) ions gets decreased.
Question 2:
In the following food chain, 100 J of energy is available to the lion. How much energy was available to the producer?
Plants —> Deer —> Lion
Answer:
10,00,000 Joules.
Question 3:
State two advantages of conserving:
(i) forests and
(ii) wild life.
Answer:
(i) Conserving forests helps in:
(a) Maintaining biodiversity of living beings.
(b) Retaining sub soil water.
(c) Also prevents the occurance of floods.
(ii) Conserving wild life helps in:
(a) Maintaining ecological balance among different species in the forest.
(b) Protecting the nature.
Question 4:
State two ways for preventing rusting of iron articles.
Answer:
(i) By applying grease and paints on the iron articles.
(ii) By coating of zinc layer over the iron articles by Galvanisation process.
Question 5:
List the properties of magnetic lines of force.
Answer:
(i) These field lines start from N pole and end at S pole of the magnet.
(ii) These lines never intersect each other.
(iii) The tangent at any point on the magnetic line gives the direction of magnetic field at that point.
(iv) The magnetic field lines of a magnet form a continuous closed loop.
Question 6:
Differentiate between metals and non-metals on the basis of their chemical
properties.
Answer:
| Metals | Non-metals |
| 1. Metals form, basic oxides with oxygen of air. | 1. Non-metals form acidic oxides with oxygen of air. |
| 2. Metals can displace hydrogen from dilute acids. | 2. Non-metals cannot displace hydrogen from dilute acids. |
| 3. Metals show displacement reactions on the basis of their reactivity series. | 3. Non-metals do not show such displacement reactions. |
Question 7:
Explain the nutrition process in Amoeba.
Answer:
Amoeba takes in the food particles with the help of its finger like projections called pseudopodia. Inside its cell a food vacuole is formed around the food particle. Inside the food vacuole, complex substances are broken down into simpler ones by the action of enzymes which are then diffused into the cell cytoplasm. The remaining undigested material is sent to the surface of the cell and thrown out.
This process of nutrition in Amoeba is called Endocytosis.
OR
Draw a labelled diagram of human heart.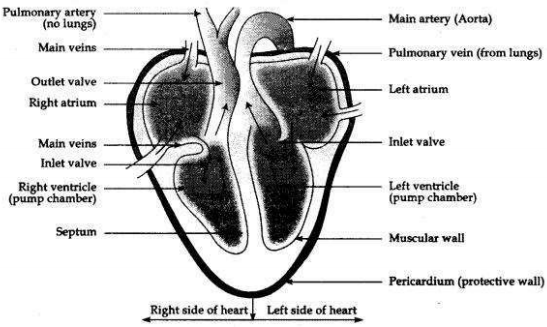
Question 8:
What is reflex arc? Draw a labelled diagram to show reflex arc on touching a
very hot object.
Answer:
Reflex Arc. The path followed during a reflex action is called reflex arc.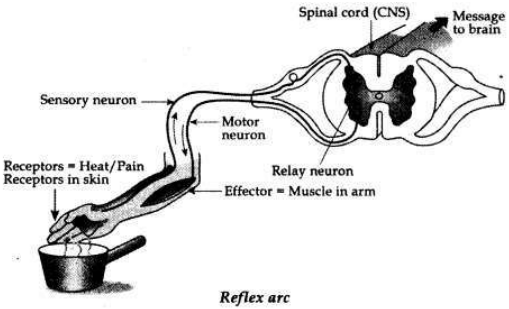
Question 9:
Show how you would connect three resistors, each of 6 Q, so that the
combination has a resistance of (a) 9 Ω, (b) 4 Ω .
Answer: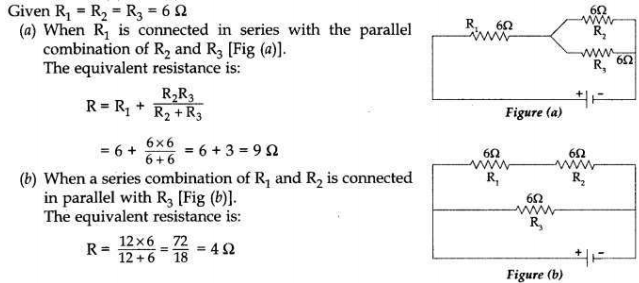
Question 10:
Draw a sketch of the pattern of field lines due to a:
(a) current flowing into a circular coil,
(b) solenoid carrying current.
Answer: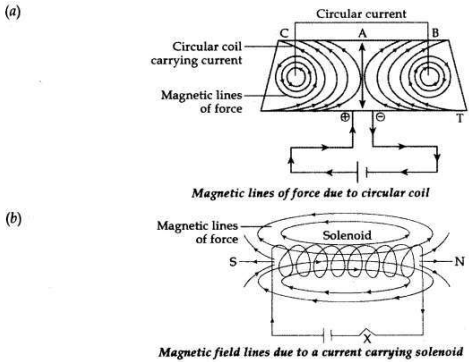
Question 11:
Write the structural formula of ethanol. What happens it is heated with excess of cone. H2SO4 acid at 443k? Write the chemical equation for the reaction, stating the role of cone. H2SO4acid in this reaction.
Answer:
Structural formula of ethanol: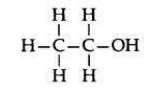
On heating ethanol with cone. H2SO4 acid ethene gas is produced.
Cone. H2SO4 acid acts as dehydrating agent which absorbs the released water.
OR
Distinguish between esterification and saponification reactions with the help of chemical equations for each. State one use of each: (t) esters and (ii) saponification process.
Answer:
Esterification. In such a reaction an alcohol reacts with a carboxylic acid in the presence of cone. H2SO4 to form sweet smelling compounds called esters.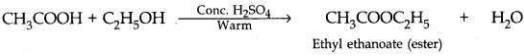
Saponification. In such a reaction an ester reacts with sodium hydroxide to form sodium salt of an acid and alcohol.![]()
It is a saponification reaction.
(i) Esters are used for making of puddings and ice creams, etc.
(ii) Saponification process is used in making of various types of soaps.
Question 12:
Na, Mg and A1 are the elements of the 3rd period of the Modern Periodic Table having group number 1, 2 and 13 respectively. Which one of these has the (a) highest valency
(b) largest atomic radius, and
(c) maximum chemical reactivity? Justify your answer stating the reason for each.
Answer:
(a) Aluminium (Al) metal has highest valency of ’3’. As it has 3 valence electrons in its outermost shell.
(b) Na atoms have largest atomic radius. As the atomic radius of atoms of the elements of same period go on decreasing as we move from left to right in a period.
(c) Na metal will show maximum chemical reactivity, as chemical reactivity among the metals goes on decreasing in the same period as we move from left to right in that period.
Question 13:
List three techniques that have been developed to prevent pregnancy. Which one of these techniques is not meant for males? How does the use of these techniques have a direct impact on the health and prosperity of a family?
Answer:
Three techniques to prevent pregnancy are:
(i) Barrier method. Use of condoms either by male or female during sexual mating.
(ii) Use of copper-T device by the females in their uterus.
(iii) Surgical method by male or female.
Use of copper-T is not meant for males.
By use of these techniques a family will have lesser number of children so their economic condition will be better. Such family will have healthy children as lesser number of children in a family will get more attention from the parents and will be cared for in a better way. It also helps to control the population.
Question 14:
If the image formed by a lens for all positions of an object placed in front of it is always erect and diminished, what is the nature of this lens? Draw a ray diagram to justify your answer. If the numerical value of the power of this lens is 10 D, what is its focal length in the Cartesian system?
Answer:
This lens is a Concave lens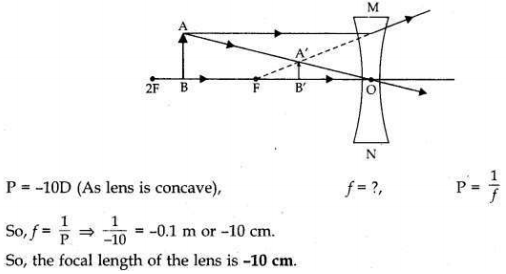
Question 15:
(a) Water is an elixir of life, a very important natural resource. Your Science teacher wants you to prepare a plan for a formative assessment activity, “How to save water, the vital natural resource”. Write any two ways that you will suggest to bring awareness in your neighbourhood, on ’how to save water’.
(b) Name and explain any one way by which the underground water table does not go down further.
Answer:
(a) Ways to bring about awareness on ’how to save’ water:
(i) We can conduct door to door campaign to make people aware about the acute shortage/scarcity of water during summer.
(ii) We can educate people on ways to save water, for example, close taps properly, use buckets of water to bathe instead of showers etc.
(iii) I can also suggest them how to store rainwater in the small park of our locality, so that it can be used during hot summer days, when availability of water is very less.
(b) We can prevent the level of underground water table to not go down further by harvesting the rainwater in the underground tanks in parks and cropfields.
Question 16:
(a) What is the importance of pH in everyday life?
(b) How are sodium hydroxide and Cl2 (Chlorene) gas produced from common
salt. What is this process called?
Answer:
(a) (i) Living organisms can survive only in a narrow range of pH change. Acidic rain water when flows into the rivers, it lowers the pH value of river water and makes the survival of acquatic life in such river water difficult. Plants require a specific pH range for their healthy growth.
(ii) Our stomach and intestines work in a specific pH range. Stomach acts in slightly acidic medium while small intestine digests the food in slightly alkaline medium.
(iii) Tooth decay starts when the pH of the mouth is lower than 5.5.
(b) When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. This process is called Chlor-alkali process because the products formed are chlorine and alkali NaOH.
2NaCl (aq) + 2H20 (1) > 2NaOH (aq) + Cl2(g) + H2 (g)
Cl2 gas is given off at the anode and H2 gas at the cathode while sodium hydroxide solution is formed near the cathode.
Question 17:
(a) Draw the structure of Neuron and explain its function.
(b) How does Phototropism occur in Plants?
Answer:
(a) Nerve cell or neuron is a functional unit of the nervous system.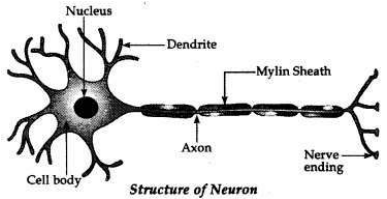
Function. The information acquired at the end of the dendritic tip of a neuron sets off a chemical reaction which creates an electrical impulse. This impulse travels from the dendrite to the cell body along the axon at its end. At the end of axon, the electrical impulse sets off the release of some chemicals, which cross the synapse and start a similar electrical impulse in a similar electrical impulse in a dendrite of the next neuron. In this way nerve impulses travel in the body, from one neuron to another till it reaches the brain or the target organ. Thus, a nervous tissue is made up of an organised network of nerve cells or nervous which are specialised in conducting information via electrical impulses from one part of the body to another.
(b) Phototropism is the response of the plant parts to the external stimulus of light. The stem of the plant grows in the direction of light while root grows away from the direction of light. This growth is controlled by the auxin hormone of the plant. The concentration of auxin stimulates the cells to grow longer on the side of the shoot (stem and branches) which is away from the light. Thus, the plant appears to bend towards light.
Question 18:
(a) Explain solar cell panel.
Give the principle of working of a windmill.
Answer:
(a) A solar cell is a device which converts solar energy directly into electricity. A group of solar cells is called a solar cell panel.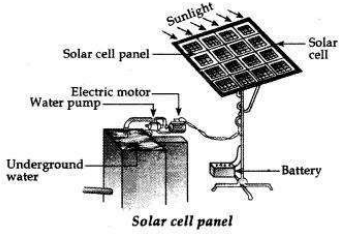
It consists of a large number of solar cells joined together in a definite pattern. It provides a lot of electric energy required by artificial satellites, water pumps, street lighting, etc.
For joining the various solar cells in a solar panel, silver wires are used because silver (metal) is the best conductor of electricity having a very low resistance, which also increases the efficiency.
(b) When the blowing wind strikes the blades of a windmill, it exerts a force on them. As a result of this force, the blades of the windmill start rotating. The rotation of the blades of the windmill causes to and fro (or up and down) motion in the crank (or connecting rod). This rotary motion is used to turn the turbines of electric generators to produce electricity and also to drive a large number of machines like water pumps and flour mills.
A large number of windmills erected over a large area are known as a wind energy farm.
Question 19:
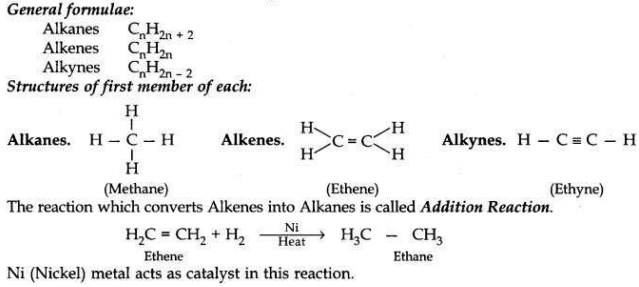
Why are certain compounds called hydrocarbons? Write the general formula for homologous series of alkanes, alkenes and alkynes and also draw the structure of the first member of each series. Write the name of the reaction that converts alkenes into alkanes and also write a chemical equation to show the necessary conditions for the reaction to occur.
Answer:
Hydrocarbons. As these compounds have only atoms of carbon and hydrogen elements in their molecules, so these are called Hydrocarbons.
Question 20:
(a) Write the functions of each of the following parts in a human female reproductive system:
(i) Ovary
(ii) Uterus
(iii) Fallopian tube
(b) Write the structure and functions of placenta in a human female.
Answer:
(a) (i) Ovary. It produces the egg cell and also produces the female hormones estrogen and progestrone.
(ii) Uterus. Implantation of zygote takes place in the uterus. Here the embryo develops into foetus and then foetus develops into the baby to be bom.
(iii) Fallopian tube. This is the site of fertilization of the egg with sperm (male gamete). It also helps in the transfer of female gamete from the ovary.
(b) Structure of placenta. It is a disc like special tissue which is embedded in the uterine wall. It has finger like villi on the embryo side and on the mother’s side there are blood spaces which surrounded the villi.
Functions of placenta. It provides a large surface area for glucose and oxygen to pass from mother’s body to the developing embryo. The developing embryo gets its nutrition from placenta. Embryo’s waste materials are passed into the mother’s blood for their removal.
Question 21:
(a) If the image formed by a mirror for all positions of the object placed in front of it is always diminished, erect and virtual, state the type of the mirror and also draw a ray diagram to justify your answer. Write one use such mirrors are put to and why?
(b) Define the radius of curvature of spherical mirrors. Find the nature and focal length of a spherical mirror whose radius of curvature is +24 cm.
Answer:
(a) This mirror is Convex mirror.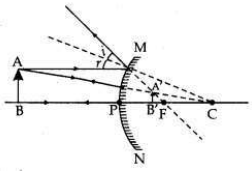
Such mirrors are used as rear view mirror in automobiles, as convex mirror gives erect, and wider range of view of objects coming behind the automobile, (b) Radius of curvature of a mirror is the distance between ’P’ point (pole) and ’C’ point (centre of curvature) of a spherical mirror.
OR
(a) A student suffering from myopia is not able to see distinctly the objects placed beyond 5 m. List two possible reasons due to which this defect of vision may have arisen. With the help of ray diagrams, explain (i) why the student is unable to see distinctly the objects placed beyond 5 m
from his eyes.
(ii) the type of the corrective lens used to restore proper vision and how this defect is corrected by the use of this lens.
(b) If, in this case, the numerical value of the focal length of the corrective lens is 5 m, find the power of the lens as per the new Cartesian sign convention.
Answer:
(a) Two possible reasons:
— The eye ball size might have got elongated.
— The eye lens is more thick than its normal thickness during its power of accomodation.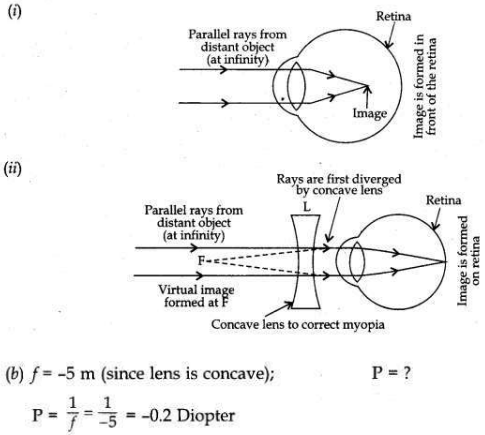
Power of correcting lens is -0.2 D and this correcting lens is concave lens.
SECTION B
Question 22:
(a) What is least count of volmeter?
(b) In a voltmenter there are 20 divisions between the ’0’ mark and 0.5 V mark. Calculate its least count.
Answer:
(a) The minimum potential difference measured by a voltmeter between the two given terminals is called the least count of that voltmeter.
(b) Two given marks of the voltmeters = 0 and 0.5 V Potential difference = 0.5 – 0 = 0.5 V No. of divisions between these two marks = 20
Leastcount=0.520=520×10[/latexs=2]=[latex]140=0.025 volt
Question 23:
(a) How is pH paper used to find the pH of a solution?
(b) The pH value of water is 7. What will be the pH value of (i) aqueous solution
of sodium hydroxide and (ii) dil. HC1.
Answer:
(a) With the help of a dropper one drop of the solution is placed on the strip of the pH paper. The colour developed on the pH paper is compared with the colour and the corresponding pH value given on the chart of the pH paper.
(b) • aqueous solution of sodium hydroxide is alkaline so its pH will be more than 7.
• dil HC1 is acidic so its pH value will be less than 7.
Question 24:
What are the precautions taken to prepare a temporary mount of a leaf peel to show its stomata?
Answer:
Precautions taken to prepare a temporary mount of a leaf peel:
(i) The epidermal peel should be taken from a freshly plucked leaf.
(ii) Always hold the slide by its edge to avoid making the slide dirty.
(iii) Always use a brush to transfer the peel from petri dish to the slide.
(iv) Curling of peel must be avoided.
(v) The peel should be mounted in the centre of the slide.
(vi) The peel should not be allowed to dry.
(vii) Always keep the cover slip gently to avoid the entry of air bubbles.
Question 25:
Mention the essential material (chemicals) to prepare soap in the laboratory. Describe in brief the test of determining the nature (acidic/alkaline) of the reaction mixture of saponification reaction.
Answer:
Chemicals needed for making of soap are—castor oil, alkaline, solution of sodium hydroxide and common salt.
After the process of saponification, the product soap is tested with litmus paper. The soap solution turns red litmus to blue, showing that soap formed during this reaction is alkaline in nature.
Question 26:
Draw in sequence (showing the four stages), the process of binary fission in Amoeba.
Answer: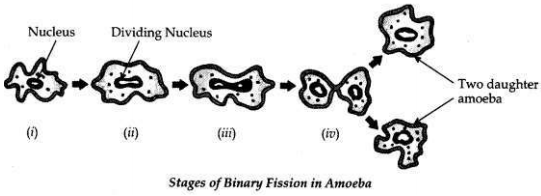
Question 27:
A student focuses the image of a candie fiame, piaced at about 2 m from a convex lens of focal length 10 cm, on a screen. After that he moves gradually the flame towards the lens and each time focuses its image on the screen.
(a) In which direction does he move the lens to focus the flame on the screen?
(b) What happens to the size of the image of the flame formed on the screen?
(c) What difference is seen in the intensity (brightness) of the image of the flame on the screen?
(d) What is seen on the screen when the flame is very close (at about 5 cm) to the lens?
Answer:
(a) He moves the lens away from the screen.
(b) The size of image formed on the screen goes on increasing.
(c) Brightness of the image of flame goes on decreasing.
(d) No distinct image of flame will be formed on the screen.