Question
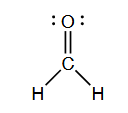
Discuss: What is the hybridization of carbon and oxygen in methanal?
▶️Answer/Explanation
Ans:A
Solution:
In methanal, also known as formaldehyde, the carbon atom is bonded to three other atoms – two hydrogen atoms and an oxygen atom. The oxygen atom is bonded to the carbon atom and has one lone pair of electrons. The electron configuration of the carbon atom in methanal is 1s$^2$2s$^2$2p$^2$. In order to form bonds with the hydrogen and oxygen atoms, the carbon atom in methanal undergoes $\mathrm{sp^2}$ hybridization. This means that the 2s orbital and two of the 2p orbitals hybridize to form three $\mathrm{sp^2}$ hybrid orbitals that are each oriented towards one of the three atoms.
Similarly, the oxygen atom in methanal is also involved in a $\mathrm{sp^2}$ hybridization. The 2s orbital and two of the 2p orbitals hybridize to form three $\mathrm{sp^2}$ hybrid orbitals that are each oriented towards the carbon atom and the two lone pairs of electrons.
Question
Discuss: the empirical formula of a hydrocarbon with $75 \%$ carbon and $25 \%$ hydrogen by mass?
A. $\mathrm{C}_3 \mathrm{H}$
B. $\mathrm{CH}_2$
C. $\mathrm{C}_2 \mathrm{H}_6$
D. $\mathrm{CH}_4$
▶️Answer/Explanation
Ans:D
Solution:
To determine the empirical formula, we need to find the simplest whole number ratio of atoms in the compound.
Given that the compound has $75 \%$ carbon and $25 \%$ hydrogen by mass, we can assume that we have $75 \mathrm{g}$ of carbon and $25 \mathrm{g}$ of hydrogen in a $100 \mathrm{g}$ sample of the compound.
Next, we need to convert the masses of carbon and hydrogen to moles by dividing by their respective atomic masses:
Number of moles of $\mathrm{C} = \frac{75 \mathrm{g}}{12 \mathrm{g/mol}} = 6.25 \mathrm{mol}$
Number of moles of $\mathrm{H} = \frac{25 \mathrm{g}}{1 \mathrm{g/mol}} = 25 \mathrm{mol}$
Now we need to find the simplest whole number ratio of carbon to hydrogen. To do this, we can divide both the number of moles of carbon and hydrogen by the smallest number of moles:
Divide both sides by $6.25 \mathrm{mol}$:
Number of moles of $\mathrm{C} = 1$
Number of moles of $\mathrm{H} = \frac{25 \mathrm{mol}}{6.25 \mathrm{mol}} = 4$
So the empirical formula of the compound is $\mathrm{CH}_4$. Therefore, the answer is D.
Question
Ammonia reacts with oxygen to produce nitrogen (II) oxide and water.
$
\_\mathrm{NH}_3(\mathrm{~g})+\ldots \mathrm{O}_2(\mathrm{~g}) \rightarrow \ldots \mathrm{NO}(\mathrm{g})+\ldots \mathrm{H}_2 \mathrm{O}(\mathrm{l})
$
What is the \(\mathrm{NH}_3: \mathrm{O}_2\) ratio in the balanced equation?
A. \(2: 5\)
B. \(4: 5\)
C. 1:1
D. \(2: 1\)
▶️Answer/Explanation
Ans:B
The balanced equation for the reaction of ammonia with oxygen to produce nitrogen (II) oxide and water is
4\mathrm{NH}_3(\mathrm{~g})+5 \mathrm{O}_2(\mathrm{~g}) \rightarrow 4 \mathrm{NO}(\mathrm{g})+6\mathrm{H}_2 \mathrm{O}(\mathrm{l})
$
So, the \(\mathrm{NH}_3: \mathrm{O}_2\) ratio in the balanced equation is \(4:5\). Therefore, the correct answer is B. \(4: 5\).
Question
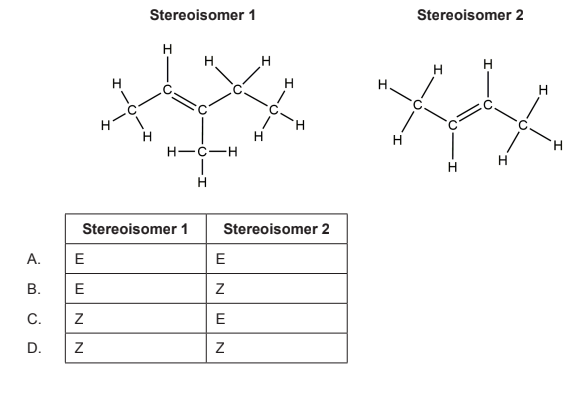
What are the E/Z designations of these stereoisomers?
▶️Answer/Explanation
Ans:
A