Question
The table shows thermal properties of water and methane.
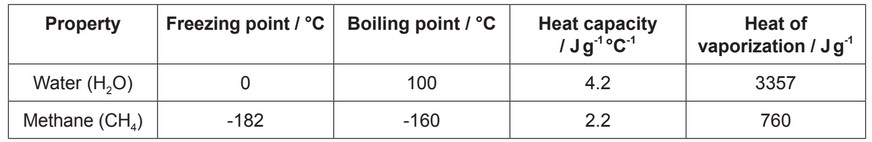
(a) Water molecules are polar and methane molecules are non-polar. Explain how this difference affects the thermal properties of these substances.
(b) Using the data in the table, deduce the reasons for methane being a gas on Earth.
(c) Water is used as a coolant in sweat. Using the data in the table, explain the reasons for methane not being as suitable as water for use as a coolant.
Answer/Explanation
Answer:
(a)
a. water forms hydrogen bonds but methane does not/hydrogen bonds form between water molecules, but are absent in methane;
b. energy needed to break hydrogen bonds/intermolecular attractions;
c. hydrogen bonds raise the freezing point/boiling point/heat capacity/heat of vaporization
(b)
a. boiling point of methane is -160°C
OR
methane is in gaseous state when temperatures are above/higher than
-160°C;
b. temperatures on Earth are always above -160°C;
(c)
a. heat of vaporization is low/heat of vaporization is only 760 J g-1
OR
methane has a lower heat of vaporization compared to water;
b. no hydrogen bonds need to be broken;
c. not enough heat removed when methane evaporates;
d. methane boils at -160 °C so would already be a gas (in/on the human body);
Question
The diagram shows water molecules as they might be arranged in liquid water and the interactions between them.
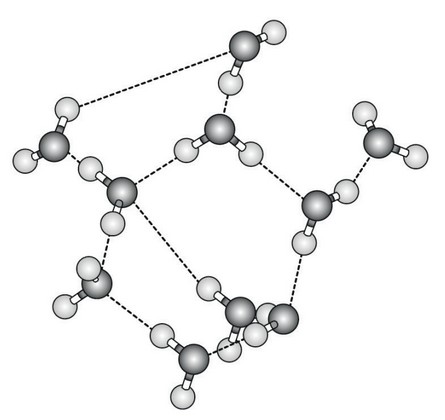
(a) (i) State how many water molecules are shown in the diagram.
(ii) Identify the interactions that are shown between the water molecules.
(b) (i) With reference to the diagram, explain how water in sweat evaporates.
(ii) Outline the reasons for secretion of sweat in humans.
Answer/Explanation
Answer:
(a) (i) 10;
(ii) hydrogen bonds/H bond;
(b)
(i)
a. heat increases molecular motion/vibration;
b. hydrogen bonds break/bonds between water molecules break;
c. water evaporation is separation of water molecules/water changes from liquid to gas/vapour;
d. heat removed from skin surface/body;
(ii)
a. cooling/removal of heat/lowering body temperature;
b. to prevent overheating
OR
to help maintain body temperature/temperature homeostasis/for
thermoregulation
OR
to keep temperature at 37 °C;
Question
Native oyster populations are decreasing where rivers meet the ocean along the northwest coast of North America. These oyster populations are being attacked by a gastropod.
It is known that oysters and gastropods have hard parts composed of calcium carbonate and that ocean acidification is increasing. Studies were carried out using juvenile oysters and gastropods to investigate the effects of acidification on the decrease in the population of oysters.
The first step was to raise oysters in two different mesocosms. One had seawater at a normal concentration of $co_{2}$ and the other had sea water with a high concentration of $co_{2}$. Gastropods were raised in two further mesocosms with normal and high $co_{2}$ concentrations respectively.
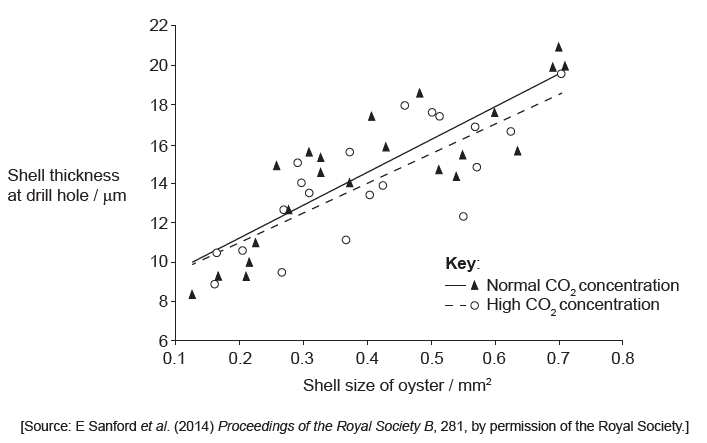
A juvenile gastropod will attack a juvenile oyster by using its tongue-like structure (radula) to drill a hole through the oyster shell. Once the hole has been drilled, the gastropod sucks out the soft flesh. Researchers investigated the shell thickness at the site of the drill hole in relation to the size of the oyster. The results are seen in this graph.
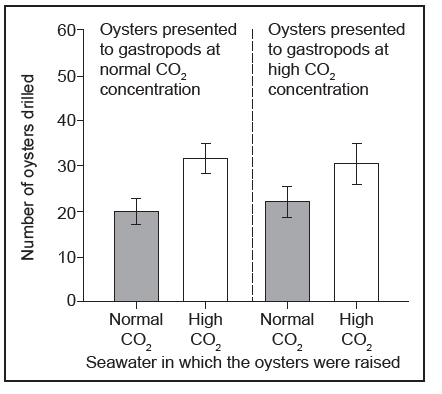
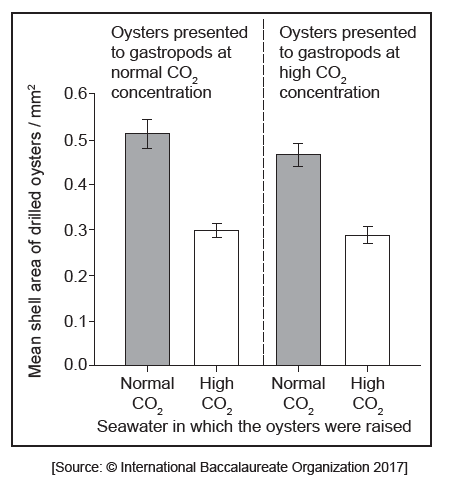
Equal numbers of oysters raised in seawater with a normal $co_{2}$ concentration and in seawater with a high $co_{2}$ concentration were then presented together to the gastropod predators in seawater with a normal $co_{2}$ concentration. The same numbers of oysters from the two groups were also presented together to the gastropods in seawater with a high $co_{2}$ concentration. The bar charts show how many of the oysters were drilled by the gastropods and the mean size of drilled oysters.
Outline how acidified sea water could affect the shells of the oyster.
Outline the trends shown in the data in the graph.
Estimate how much smaller drilled oysters raised in seawater at a high $co_{2}$ concentration were than drilled oysters raised in seawater at a normal $co_{2}$ concentration.
Deduce from the data in the bar charts which factors were and were not correlated significantly with the number of oysters drilled by the gastropods.
Suggest reasons for the differences in the numbers of oysters drilled, as shown in the bar charts.
The radula in a gastropod is hard but not made of calcium carbonate. Outline how this statement is supported by the drilling success of the gastropods in seawater with normal or high $co_{2}$ concentrations.
Using all the data, evaluate how $co_{2}$ concentrations affect the development of oysters and their predation by gastropods.
▶️Answer/Explanation
Markscheme
a. Shells might dissolve/deteriorate / become smaller/thinner/weaker / OWTTE
OR
shell formation reduced / more difficult
b. a. positive correlation between shell thickness and shell size
OR
as shell thickness increases, shell size «also» increases
b. (positive correlation) occurs at two different $\mathrm{CO}_2$ concentrations / both high and normal concentrations
c. trend for thickness is «slightly» lower with high $\mathrm{CO}_2$
c. «approximately» $0.2 \mathrm{~mm}^2$
OR
«approximately» $40 \%$ «smaller»
unit required
d.i. a.) significant factor: concentration of $\mathrm{CO}_2$ in which oysters were raised
b. ) insignificant factor: concentration of $\mathrm{CO}_2$ at which oysters were presented to gastropods
d.ii.a. ) (because) shells are thinner/smaller when the oyster is raised in high $\mathrm{CO}_2$ /lower $\mathrm{pH}$
OR
d.ii.)
«because» lower pH/higher acidity prevents/reduces deposition of calcium carbonate
b. gastropods target smaller/thinner-shelled oysters more
c. gastropods can eat/drill thin-shelled/smaller oysters at a faster rate (and move onto another)
d. eating smaller oysters «from high $\mathrm{CO}_2$ environments» means given population of gastropods require more oysters for same food intake
d.iiia. data shows that similar numbers are drilled regardless of conditions
b. since radulas are not affected by acidification
OR
radulas not made of calcium carbonate so (remain) strong/successful at drilling
e. a. the data/trend lines indicate that a higher $\mathrm{CO}_2$ concentration diminishes the shell thickness, making gastropod predation more successful
OR
the bar graphs suggest that oysters raised in a higher $\mathrm{CO}_2$ concentration are smaller, making gastropod predation more successful
b. $\mathrm{CO}_2$ concentrations «during feeding» do not change the occurrence of drilling/predation «by gastropods»
c. «limitation» no information about how exaggerated the $\mathrm{CO}_2$ concentrations were
OR
«limitation» no information about numbers of gastropods used «in each setting»
Question
The figure represents a water molecule.
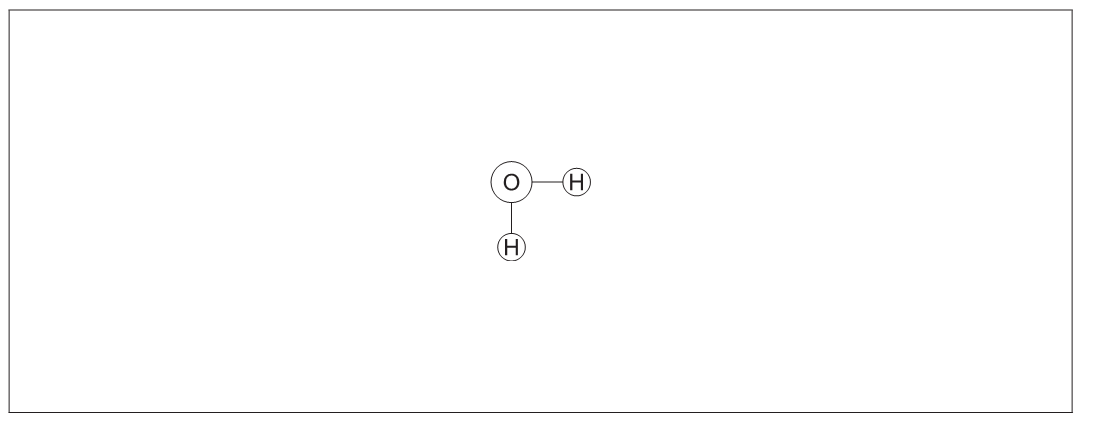
a. Draw a second water molecule to show how bonds can form between water molecules, including the name of the bond.[2]
b. Water has important solvent properties. Explain these properties using an example to illustrate your answer. [3]
▶️Answer/Explanation
Markscheme
a.
a. similar water molecule drawn with oxygen on one molecule facing hydrogen on the other water molecule
b. one hydrogen bond drawn as a dotted/dashed line between the two water molecules and labelled
O and H do not have to be labelled but must be positioned correctly
eg :
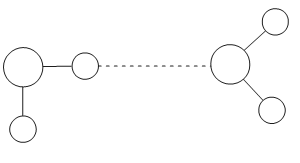
Can get this mark even if atoms incorrect
a. water molecule is polar
OR
water has «weak» positive and negative charges
c. water forms hydrogen bonds with polar substances
d. positive/hydrogen side/pole of water attracted to negative ions
OR
negative/oxygen side/pole attracted to positive ions
e. glucose/other example dissolves because it is polar
OR
sodium chloride/other example dissolves because ions are attracted to water
Question
a. (i) Distinguish between the thermal properties of water and methane.[6]
(ii) Explain the reasons for the unique thermal properties of water. [4]
▶️Answer/Explanation
Markscheme
(i)
Boiling point of water is greater than methane
Melting point of water is greater than methane
Latent heat of vaporization of water is greater than methane
OR
specific heat capacity of water is greater than methane
(ii)
Water is polar
OR
O atom more negative
OR
H atoms more positive
This causes «strong» hydrogen bonds to form between the molecules
Which require more/high amount of energy to break
Which increases the melting/boiling/latent heat properties
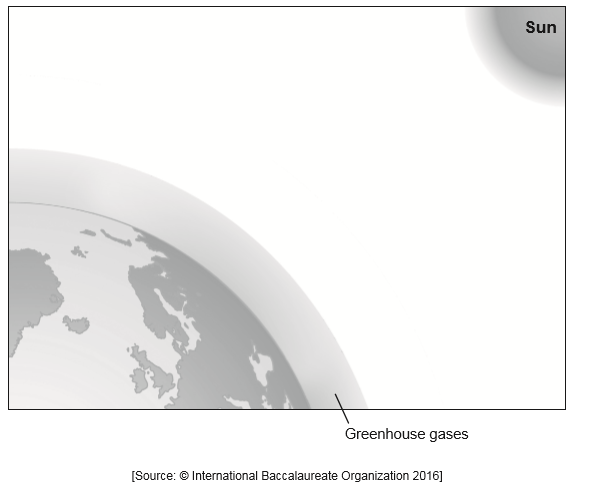
Short wave radiation/UV «shown as» having its origin in the Sun gives off light as short radiation
Short wave radiation/UV «shown as» passing through the greenhouse gases «some reflected»
Some short wave radiation/UV is absorbed by the Earth and some is reflected
The reflected radiation is long wave radiation «reflected as heat»
Long wave radiation/IR «shown as» being unable to pass through/being absorbed/reflected by the greenhouse gases
Award marks for diagrammatic explanations of these marking points.
Accept UV and IR as long as they are drawn with the correct wavelength.
Question
a.Describe the properties of water that make it a useful component of blood. [4]
b. Explain the relationship between structure and function of arteries, capillaries and veins. [8]
c. Outline how leucocytes defend the body against pathogens. [6]
▶️Answer/Explanation
Markscheme
a.
a.water is a polar molecule / hydrogen bonding;
b. (makes it) (versatile) solvent;
c. example of dissolved substance (eg salts/proteins or other example);
d. (water is) fluid/liquid at body temperature;
e. example of transported material (eg nutrients/metabolic wastes/gases/hormones/blood cells or other example);
f. high heat capacity/specific heat allows water to carry heat without warming up;
g. (allows) blood to move heat (for warming/cooling/homeostasis);
Arteries: [3 max]
a. thick walls to withstand high pressure/maintain blood flow/pressure;
b. collagen fibres/elastic fibres/connective tissue (in outer layer) give wall strength/flexibility/ability to stretch and recoil;
c. (smooth) muscle layer (contracts) to maintain pressure;
d. narrow lumen maintains high pressure;
e. smooth endothelium for efficient transport/reduced friction;
Capillaries: [3 max]
f. wall has one layer of cells allowing (fast) diffusion of substances;
g. pores to allow lymphocytes/plasma to exit / to increase permeability;
h. extensive branching increases surface area for exchange of materials;
i. small diameter allows them to fit between cells/perfuse tissue;
j. narrow diameter increases oxygen diffusion from RBC;
Veins: [3 max]
k. thin walls allow (skeletal) muscles to exert pressure on veins;
l. thin outer layer of collagen/elastic/muscle fibres provide structural support;
m. wide lumen allows great volume of blood to pass;
n. valves prevent backflow;
NB Every structure requires a function for the mark.
a. leucocytes/phagocytes/macrophages can recognize pathogens/foreign matter;
b. (phagocytes) engulf pathogens by endocytosis/phagocytosis;
c. migration to tissues/squeezing out of capillaries;
d. each pathogen has specific antigens;
e. leukocytes/lymphocytes produce antibodies by reacting to specific antigen/ pathogens;
f. antibody joins to (specific) antigen inactivating/destroying them;
g. lymphocyte makes a clone/copies itself;
h. thus increasing the total number of (specific) antibodies;
Question
a. Outline the bonding between DNA nucleotides.[2]
b. Explain how chemical bonding between water molecules makes water a valuable coolant in living organisms.[2]
c.Describe the movement of water across membranes.[2]
d. Outline the role of water in photosynthesis.[2]
▶️Answer/Explanation
Markscheme
hydrogen bonds between nucleotides of opposite strands/complementary bases/adenine and thymine and cytosine and guanine;
covalent bonds between nucleotides within strands/between sugar/deoxyribose and phosphate;
hydrogen bonding between water molecules;
breaking (hydrogen bonds) needs/removes energy/heat;
hydrogen bonds must break when water evaporates/vaporizes;
osmosis / moves passively;
from regions of low solute/high water potential/concentration to high solute concentration / low water potential/concentration;
passes through protein channels/aquaporins/selectively-permeable membrane;
water molecules undergo photolysis/are split by light energy;
forms oxygen as a by-product;
hydrogen helps power the fixation of carbon (into organic molecules);
Question
a.State one role in living organisms for each of the following: sulfur, calcium, phosphorus and iron.[4]
b.Outline the role of condensation and hydrolysis in the relationship between fatty acids, glycerol and triglycerides.[6]
c.Explain the relationship between the properties of water and its uses in living organisms as a coolant, a medium for metabolic reaction and a transport medium. [8]
▶️Answer/Explanation
Markscheme
a.sulfur: (structural element in some) amino acids/proteins/enzymes;
calcium: (structural element in) bones/teeth/shells / signal for cellular processes/ neurotransmitter release/muscle contraction/electrical conduction system of the heart/blood clotting;
phosphorus: (structural element in) ATP/DNA/RNA/phospholipids/bones;
iron: carries oxygen / formation of hemoglobin/myoglobin/cytochrome / cofactor of enzymes;
hydrolysis: [3 max]
when larger molecules are broken to smaller molecules/subunits;
with the addition of water;
fatty acids produced by the hydrolysis of fats/triglycerides;
breaking of ester bonds;
with release of glycerol;
condensation: [3 max]
when molecules/subunits are joined to form a larger molecule;
water formed/removed;
fatty acids linked to glycerol;
up to three fatty acids can be linked to each glycerol;
through formation of ester bonds;
water is a polar molecule;
oxygen has a partial negative charge / hydrogen has a partial positive charge;
hydrogen bonds form between adjacent water molecules;
water remains liquid over wide range of temperatures/0 to 100 °C ;
moderates temperature fluctuation / stable environment;
accurate reference to specific heat;
sweating/evaporation cools organisms;
accurate reference to high heat of vaporization;
polarity makes water a good/universal solvent for polar/ionic substances;
(all) metabolic reactions of cells take place in (aqueous) solutions;
blood/xylem/phloem transport solutes in water;
cohesive properties allow capillary action/transpiration stream/water column in xylem;
Question
a. Draw a labelled diagram to show the fluid mosaic structure of a plasma membrane, indicating the hydrophilic and hydrophobic regions[5]
b.Distinguish between active and passive movements of materials across plasma membranes, using named examples.[4]
c. Explain how the properties of water are significant to living organisms. [9]
▶️Answer/Explanation
Markscheme
a.Award [1] for each structure clearly drawn and correctly labelled.
phospholipid bilayer – with head and tails;
hydrophilic/phosphate/polar heads and hydrophobic/hydrocarbon/fatty acid/ non-polar tails labelled;
integral protein – embedded in hydrophobic region of the phospholipid bilayer;
protein channel – integral protein showing clear channel/pore;
peripheral protein – on the surface;
glycoprotein with carbohydrate attached on outside;
cholesterol – shown embedded in bilayer; thickness indicated (10 nm); (allow 7 nm to 13 nm)
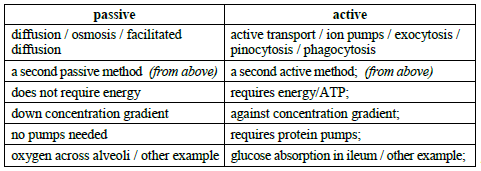
Both the passive and active movements must be contrasted to receive a mark.
Award [3 max] if no examples are given.
Responses do not need to be shown in a table format.
water is transparent / light passes through water;
this allows organisms to live below the surface / plants to photosynthesize;
hydrogen bonds between water molecules make water cohesive;
this gives water a high surface tension allowing animals to live on the surface / maintains lung structure (pleural membranes);
helps in water movement through plants/transpiration;
water has a high latent heat of vaporization / OWTTE;
evaporation/sweating/transpiration leads to cooling;
water has a high specific heat capacity / OWTTE;
this provides a stable environment for water organisms;
water is a universal solvent; can transport materials around organisms/plants/animals;
can be a solvent for chemical reactions in organisms;
ice is less dense than water / water has a maximum density at 4°C surface (pond/lake/ocean) freezes first, allowing organisms to survive in the water below;
Accept hydrogen bonds between water and other substance makes water adhesive from AHL.