Question
Solid ionic compounds form crystal lattices.
(a) Enthalpy of solution, enthalpy of hydration and lattice enthalpy are related in an energy cycle.
(i) Annotate the energy cycle for the enthalpy of solution of solid magnesium chloride, \(MgCl_2\) (s), by naming the processes A, B and C and completing the boxes. Include state symbols.

(ii) Calculate the enthalpy of solution for magnesium chloride, \(MgCl_2\). Use sections 18 and 20 of the data booklet.
(b) Explain why the lattice enthalpy of barium chloride, \(BaCl_2\), is lower than that of magnesium chloride.
(c) Cobalt also forms chlorides with the formula \(CoCl_2\).
(i) State the full electron configuration of the cobalt(II) ion, \(Co^{2+}\).
(ii) Hydrated cobalt(II) ions, \(Co(H_2O)_6^{2+}\), are pink. Describe the interaction between the cobalt ion and a water molecule in terms of the type of bond and how this bond is formed.
Type of bond:
How the bond forms:
\(CoCl_4^{2−}\) ions are blue.
(iii) Explain why the different ligands cause different coloured complexes.
Answer/Explanation
Answer:
(a) (i) 
correct boxes
A: enthalpy of solution / \(\delta \)Hsolution / \(\delta \)Hsol
AND
B: lattice enthalpy / \(delta \)Hlattice
AND
C: enthalpy of hydration / \(\delta \)Hhydration
(ii) «\(\delta\)Hsolution = \(\delta \)Hlattice + \(\delta \)Hhydration
= 2540 + (−1963) + 2(−359) =»
−141 «kJ \(mol^{−1}\) »ü
(b) ionic radius \(Ba^{2+}\) is greater than that of \(Mg^{2+}\)
weaker attraction between «\(Ba^{2+}\) and \(Cl^−\) » ions
(c) (i) \(1s^2\) \(2s^2\) \(2p^6\) \(3s^2\) \(3p^6\) \(3d^7\)
(ii) Type of bond:
coordinate/dative/covalent
How the bond forms:
oxygen/water molecule /ligand donates e− pair to cobalt«(II) ion»
(iii) magnitude of ∆E/energy gap between split d-orbitals differs «according to ligand»
∆E/energy gap determines the wavelength of light absorbed/colour «of complex»
OR
energy absorbed by electrons «to transition/to be promoted to higher levels»
corresponds to different wavelengths of light/colour
Question
Chlorine undergoes many reactions.
(a) (i) State the full electron configuration of the chlorine atom. [1]
(ii) State, giving a reason, whether the chlorine atom or the chloride ion has a larger radius. [1]
(iii) Outline why the chlorine atom has a smaller atomic radius than the sulfur atom. [2]
(iv) The mass spectrum of chlorine is shown.
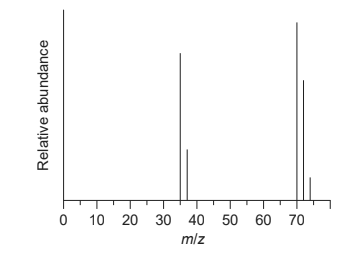
Outline the reason for the two peaks at m/z = 35 and 37. [1]
(v) Explain the presence and relative abundance of the peak at m/z = 74. [2]
(b) 2.67 g of manganese(IV) oxide was added to 200.0 cm3 of 2.00 mol dm–3 HCl.
MnO2 (s) + 4HCl (aq) → Cl2 (g) + 2H2O (l) + MnCl2 (aq)
(i) Calculate the amount, in mol, of manganese(IV) oxide added. [1]
(ii) Determine the limiting reactant, showing your calculations. [2]
(iii) Determine the excess amount, in mol, of the other reactant. [1]
(iv) Calculate the volume of chlorine, in dm3, produced if the reaction is conducted at standard temperature and pressure (STP). Use section 2 of the data booklet. [1]
(v) State the oxidation state of manganese in MnO2 and MnCl2. [2]
MnO2:
MnCl2:
(vi) Deduce, referring to oxidation states, whether MnO2 is an oxidizing or reducing agent. [1]
(c) Chlorine gas reacts with water to produce hypochlorous acid and hydrochloric acid.
Cl2 (g) + H2O (l) HClO (aq) + HCl (aq)
(i) Hypochlorous acid is considered a weak acid. Outline what is meant by the term weak acid. [1]
(ii) State the formula of the conjugate base of hypochlorous acid. [1]
(iii) Calculate the concentration of H+ (aq) in a HClO (aq) solution with a pH = 3.61. [1]
(d) (i) State the type of reaction occurring when ethane reacts with chlorine to produce chloroethane. [1]
(ii) Predict, giving a reason, whether ethane or chloroethane is more reactive. [1]
(iii) Explain the mechanism of the reaction between chloroethane and aqueous sodium hydroxide, NaOH (aq), using curly arrows to represent the movement of electron pairs.[3]
(iv) Ethoxyethane (diethyl ether) can be used as a solvent for this conversion. Draw the structural formula of ethoxyethane. [1]
(v) Deduce the number of signals and chemical shifts with splitting patterns in the 1H NMR spectrum of ethoxyethane. Use section 27 of the data booklet. [3]
(e) CCl2F2 is a common chlorofluorocarbon, CFC.
(i) Calculate the percentage by mass of chlorine in CCl2F2. [2]
(ii) Comment on how international cooperation has contributed to the lowering of CFC emissions responsible for ozone depletion. [1]
(iii) CFCs produce chlorine radicals. Write two successive propagation steps to show how chlorine radicals catalyse the depletion of ozone. [2]
Answer/Explanation
Ans:
1. a i 1s22s22p63s23p5 Do not accept condensed electron configuration.
1. a ii Cl − AND more «electron–electron» repulsion Accept Cl − AND has an extra electron.
1. a iii
Cl has a greater nuclear charge/number of protons/Zeff «causing a stronger pull on the outer electrons» ✔
same number of shells
OR
same «outer» energy level
OR
similar shielding ✔
1. a iv «two major» isotopes «of atomic mass 35 and 37»
1. a v
«diatomic» molecule composed of «two» chlorine-37 atoms ✔
chlorine-37 is the least abundant «isotope»
OR
low probability of two 37Cl «isotopes» occurring in a molecule ✔
1. b i « » 0.0307 «mol»
1. b ii
«nHCl = 2.00 mol dm-3 x 0.2000 dm3» = 0.400 mol
« =» 0.100 mol AND MnO2 is the limiting reactant
Accept other valid methods of determining the limiting reactant in M2.
1. b iii
«0.0307 mol × 4 = 0.123 mol»
«0.400 mol – 0.123 mol =» 0.277 «mol»
1. b iv «0.0307 mol × 22.7 dm3 mol−1 =» 0.697 «dm3» Accept methods employing pV = nRT.
1. b v
MnO2: +4
MnCl2: +2
1. b vi oxidizing agent AND oxidation state of Mn changes from +4 to +2/decreases
1. c i partially dissociates/ionizes «in water»
1. c ii ClO–
1. c iii «[H+] = 10−3.61 =» 2.5 × 10−4 «mol dm−3»
1. d i «free radical» substitution/SR Do not accept electrophilic ornucleophilic substitution.
1. d ii
chloroethane AND C–Cl bond is weaker/324 kJmol−1 than C–H bond/414 kJmol−1
OR
chloroethane AND contains a polar bond ✔
1. d iii
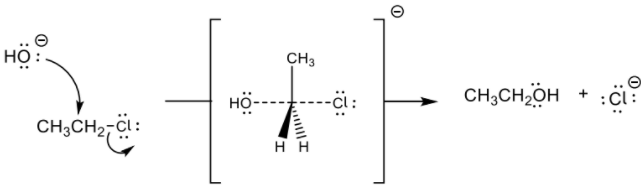
curly arrow going from lone pair/negative charge on O in −OH to C ✔
curly arrow showing Cl leaving ✔
representation of transition state showing negative charge, square brackets and partial bonds ✔
1. d iv 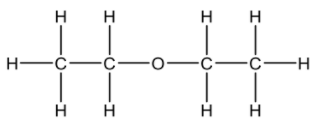 / CH3CH2OCH2CH3
/ CH3CH2OCH2CH3
1. d v
2 «signals» ✔
0.9−1.0 AND triplet ✔
3.3−3.7 AND quartet ✔
1. e i
«M (CCl2F2) =» 120.91 «g mol−1»
1. e ii
Any of:
research «collaboration» for alternative technologies «to replace CFCs»
OR
technologies «developed»/data could be shared
OR
political pressure/Montreal Protocol/governments passing legislations ✔
1. e iii
O3 + Cl∙→ O2 + ClO∙
ClO∙ + O∙→ O2 + Cl∙
OR
ClO∙ + O3 → Cl∙ + 2O2