Question:
What did you notice about the rate of corrosion for the metals tested?
▶️Answer/Explanation
Ans: When compared to many reactions that occur in a school laboratory, the rate of corrosion of metals in water is relatively slow.
Question:
Place them in order from most corroded to least corroded.
▶️Answer/Explanation
Ans: From qualitative observations, an order from most corroded to least corroded can be determined. Generally the more reactive metals will show greater evidence of corrosion.
Question:
What did you observe in the test tube with the iron and boiled water?
▶️Answer/Explanation
Ans: No corrosion of iron will be evident in the test tube with boiled water.
Question:
Explain any differences in the reactions between the iron in the boiled water compared to that in the normal distilled water. What does this tell you about the required conditions for corrosion of a metal?
▶️Answer/Explanation
Ans: Distilled water is pure molecular water with no dissolved salts. However, dissolved oxygen is still present. When you boil water, this dissolved oxygen is released into the atmosphere. From this we can infer that the presence of oxygen is a required condition for the corrosion of the metal.
Question:
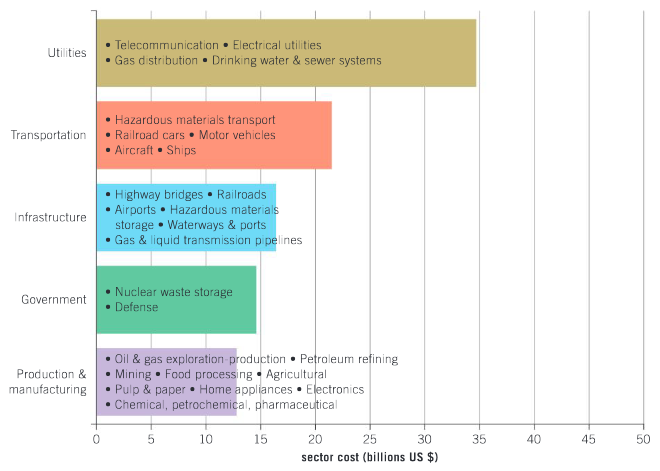
Which sector of the economy is impacted the most by corrosion?
▶️Answer/Explanation
Ans: Utilities sector.
Question:
In a group, brainstorm why this sector is most affected by corrosion, then summarize the main reasons.
▶️Answer/Explanation
Ans: Students to read, think about and discuss the data presented.
Question:
The next highest cost sector is transportation. Explain the possible reasons for this.
▶️Answer/Explanation
Ans: Transportation has a heavy workload with constant use and in a variety of corrosive environments. These could include exposure to salt water, surfaces which have been salted to prevent ice forming, stone chips off paint work – all initiate corrosion.
Question:
Manufacturers are investing in technology to reduce the amount of corrosion in motor vehicles. What strategies are they using?
▶️Answer/Explanation
Ans: Discussions may focus on the application of corrosion inhibitors to metal surfaces, the replacement of corrosion prone parts with plastic or synthetic components that are corrosion resistant, the increasing use of aluminium which is more resistant to corrosion.
Question:
Ships are protected from corrosion by a sacrificial anode. Research this term. What are the most common metals used for these anodes?
▶️Answer/Explanation
Ans: A sacrificial anode is a metal that will be preferentially corroded instead of iron. These metals are normally found higher on the reactivity series than iron, such as magnesium, aluminium and zinc. They protect ships made from steel as these metals are corroded first.
Question:
How might you prevent the formation of carbon monoxide during the combustion of alkanes?
▶️Answer/Explanation
Ans: Ensure that the combustion reaction has a constant supply of oxygen.
Question:
Draw the structural formula for each alcohol in your table.
▶️Answer/Explanation
Ans: 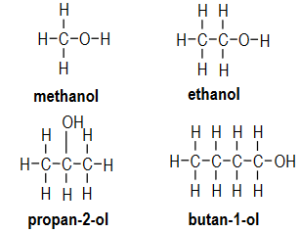
Question:
Analyze the qualitative data collected and then explain with reasons why each reaction is either complete or incomplete combustion.
▶️Answer/Explanation
Ans: Qualitative data collected should reveal differences in the colour of the flame. A yellow flame that gives off dark coloured smoke is evidence of incomplete combustion. A clean blue flame is evidence of complete combustion.
Question:
What is the composition of the substance that appeared on the test tube?
▶️Answer/Explanation
Ans: Carbon, often referred to as soot.
Question:
How does increasing the carbon-length of alcohols affect the type of combustion reaction that occurs?
▶️Answer/Explanation
Ans: Short carbon–length alcohols are more volatile and tend to undergo complete combustion more readily. As the carbon–length of the alcohol increases, the amount of oxygen required for combustion increases. There is a tendency for long carbon–length alcohols to undergo incomplete combustion.
Question:
Write balanced chemical equations for each combustion reaction. Base the products for each reaction on the conclusions you reached about the nature of the combustion reaction.
▶️Answer/Explanation
Ans: If the reaction is complete combustion, the equation will follow the general form of the combustion of methanol:
2CH3OH(l) + 3O2(g) → 2CO2(g) + 4H2O(g)
If the reaction is incomplete combustion, the equation will follow the general form of the combustion of butan–1–ol:
2C4H9OH(l) + 😯2(g) → 8CO(g) + 10H2O(g)
Question:
Why does magnesium replace hydrogen when it reacts with hydrochloric acid?
▶️Answer/Explanation
Ans: Magnesium is higher in the reactivity series than hydrogen.
Question:
Would pure silver jewelry react with an acid?
▶️Answer/Explanation
Ans: No, because silver is lower in the reactivity series than hydrogen.
Question:
Predict how the reaction between lead and hydrochloric acid would compare to the reaction between magnesium and hydrochloric acid. Describe how your observations of the two reactions would differ.
▶️Answer/Explanation
Ans: Lead and magnesium will both react with hydro–chloric acid, but magnesium is more reactive, so the rate of reaction is higher. This can be con– firmed by observing the number of bubbles (hydrogen gas) produced in a given period. The reaction between magnesium and hydrochloric acid will produce a greater number of bubbles than the reaction between lead and hydrochloric acid.
Question:
Look back at the question earlier in the chapter about sacrificial anodes. What do you notice about their position in the reactivity series?
▶️Answer/Explanation
Ans: Metals commonly used as sacrificial anodes, such as magnesium, aluminium and zinc, are higher than iron in the reactivity series.
Question:
Discuss the differences in the level of reactivity of each metal.
▶️Answer/Explanation
Ans: Conclusions drawn about the level of reactivity of each metal should be based on qualitative observations and supported by the relevant scientific context.
Question:
Order the reactions from vigorous to unreactive based on your qualitative observations.
▶️Answer/Explanation
Ans: The order of reactivity of metals based on experimental evidence can be compared to the reactivity series found on page 204.
Question:
Describe the appearance of a fresh piece of zinc metal.
▶️Answer/Explanation
Ans: A fresh piece of zinc metal has a shiny, metallic luster.
Question:
Describe what happens when you dip the zinc metal into copper(II) sulfate solution. Explain the type of reaction you think has occurred.
▶️Answer/Explanation
Ans: When you dip a piece of zinc metal into a copper(II) sulfate solution, there is an immediate reaction and a dark brown/red colored layer is formed on the zinc metal. This is evidence of a redox reaction.
Question:
How does the zinc’s appearance change after it has been in the solution for 15 minutes?
▶️Answer/Explanation
Ans: After 15 minutes, a large amount of solid has plated onto the zinc metal. The original deep blue colour of copper(II) sulfate has faded.
Question:
Write a balanced chemical equation to describe the reaction.
▶️Answer/Explanation
Ans: CuSO4(aq) + Zn(s) → Cu(s) + ZnSO4(aq)
Question:
For each of the following ionic equations:
a) Balance the equation so the net charge is the same on each side of the equation
b) Construct the two half-equations from the ionic equation
c) Balance the charges for each half-equation by adding electrons
d) Identify the species that is being oxidized and the species being reduced.
Cu2+ + Mg ➝ Cu + Mg2+
Al + Fe3+ ➝ Al3+ + Fe
Ag+ + Cd ➝ Cd2+ + Ag
Sn + Pb2+ ➝ Sn4+ + Pb
▶️Answer/Explanation
Ans: Reaction 1
Cu2+ + Mg → Cu + Mg2+
Cu2+ + 2e– → Cu
Mg → Mg2+ + 2e–
Cu2+ ion is being reduced and Mg atom is being oxidized.
Reaction 2
Al + Fe3+ → Al3+ + Fe
Fe3+ + 3e– → Fe
Al → Al3+ + 3e–
Fe3+ ion is being reduced and Al atom is being oxidized.
Reaction 3
2Ag+ + Cd → Cd2+ + 2Ag
2Ag+ + 2e– → 2Ag
Cd → Cd2+ + 2e–
Ag+ ion is being reduced and Cd atom is being oxidized.
Reaction 4
Sn + 2Pb2+ → Sn4+ + 2Pb
2Pb2+ + 4e– → 2Pb
Sn → Sn4+ + 4e–
Pb2+ ion is being reduced and Sn atom is being oxidized.
Question:
Construct the half-equations for the rusting of iron.
▶️Answer/Explanation
Ans: 4Fe(s) + 3O2(g) → 2Fe2O3(s)
Fe → Fe2+ + 2e–
½O2 + 2e– → O2–
The second step in this process to form hydrated iron oxide is a multi–step process and beyond requirements of this curriculum.
Summative assessment
Question:
Combustion reactions are an example of an oxidation reaction.
a) What is a combustion reaction? Illustrate your answer with a word equation or balanced chemical equation.
▶️Answer/Explanation
Ans: A combustion reaction is a combination of a substance with an oxidant, normally oxygen, to produce heat and new products. An example is the combustion of methane gas with oxygen to produce carbon dioxide, water and heat.
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
b) Give three examples of combustion reactions that play a role in your daily life.
▶️Answer/Explanation
Ans: Examples may include cellular respiration, the combustion of fuels in transport vehicles, the burning of coal in power generation plants that provide electricity to your home.
c) Explain the difference between complete and incomplete combustion reactions.
▶️Answer/Explanation
Ans: Complete combustion occurs when there is sufficient or an excess of oxygen for the reaction to occur; products include carbon dioxide and water; incomplete combustion is the result of a lack of sufficient oxygen; products include elemental carbon or carbon monoxide and water.
Question:
A student performed a series of reactions between a metal and solution and recorded whether or not there was a reaction. Consider the results and answer the following questions.
a) Identify the most reactive metal.
▶️Answer/Explanation
Ans: The most reactive metal is potassium; as it reacted with all other metal salts in the experiment.
b) Iron is less reactive than aluminium. Provide evidence to justify this statement.
▶️Answer/Explanation
Ans: Aluminium is able to displace iron ions from solution in the reaction between aluminium and iron(III) chloride; iron is not able to displace aluminium ions from solution in the reaction between iron and aluminium chloride.
c) State which is the least reactive metal and give reasons for your choice.
▶️Answer/Explanation
Ans: The least reactive metal is silver; which was unable to displace any of the cations from solution in all five reactions.
d) List the metals in order of most reactive to least reactive.
▶️Answer/Explanation
Ans: Potassium > magnesium > aluminium > iron > copper > silver.
Question:
The single replacement reactions in question 2 are examples of a redox reaction. For each of the reactions listed below:
a) write the balanced chemical equation for the reaction
b) write the two half-equations for the reaction
c) identify the species being oxidized and the species being reduced.i) Silver nitrate and magnesium
ii) Aluminium and copper(II) nitrate
iii) Potassium and magnesium iodide
iv) Iron and copper(II) nitrate (hint: iron(III) is one of the products)
▶️Answer/Explanation
Ans: i) a) 2AgNO3(aq) + Mg(s) → Mg(NO3)2(aq) + 2Ag(s)
(One mark for correct reactants and one mark for correct products)
b) 2Ag+(aq) + 2e– → 2Ag(s)
Mg(s) → Mg2+ (aq) + 2e–
c) Ag+ is reduced; Mg is oxidized.
ii) a) 3Cu(NO3)2(aq) + 2Al(s) → 2Al(NO3)3(aq) + 3Cu(s)
(One mark for correct reactants and one mark for correct products)
b) 3Cu2+(aq) + 6e– → 3Cu(s)
2Al(s) → 2Al3+(aq) + 6e–
c) Cu2+ is reduced; Al is oxidized.
iii) a) MgI2(aq) + 2K(s) → 2KI(aq) + Mg(s)
(One mark for correct reactants and one mark for correct products)
b) Mg2+(aq) + 2e– → Mg(s)
2K(s) → 2K+ (aq) + 2e–
c) Mg2+ is reduced; K is oxidized.
iv) a) 3Cu(NO3)2(aq) + 2Fe(s) → 2Fe(NO3)3(aq) + 3Cu(s)
(One mark for correct reactants and one mark for correct products)
b) 3Cu2+(aq) + 6e– → 3Cu(s)
2Fe(s) → 2Fe3+(aq) + 6e–
c) Cu2+ is reduced; Fe is oxidized.
Identifying the reactivity of a metal from experimental data
The reaction between a metal and an acid produces a soluble salt and hydrogen gas. The rate of production of hydrogen gas is a consequence of the reactivity of the metal present during the reaction. To ensure that the reaction rate is a reflection of the reactivity of the metal, you will need to select the dependent and independent variable, and design a method that controls all other variables.
Question:
Design an experiment to investigate the effect the reactivity of a metal has on the initial rate of production of hydrogen gas. Use the following points for guidance.
▶️Answer/Explanation
Ans: Design should include clear statement of:
- independent and dependent variables
- rationale for the method and practical details, including
- correct names of apparatus and volume
- amount or mass of metal used and concentration of acid used
- consideration of safety, ethical and environmental issues
- description of the step–by–step methodology for the investigation, including how the variables are controlled
- description of how qualitative observations will be recorded
- identification of quantitative data that will be recorded and the design of data tables to present this information
Marks awarded on a scale from 0 marks for a completely inadequate design to 5 marks for an exemplary design.
Question:
Explain the importance of the control variables in this experiment.
▶️Answer/Explanation
Ans: The reactivity of the metal is being examined, therefore, the mass or amount of moles of the metal must remain constant so that it does not affect the amount of hydrogen being produced; the concentration and volume of hydrochloric acid used in each experiment must also remain constant so that it does not affect the rate of the reaction; the temperature of the reaction mixture must remain constant so that this variable does not affect the rate of the reaction.
Question:
Explain how the method you have designed enables you to examine the initial rate of reaction and hence make a comparison between the reactivity of the metals.
▶️Answer/Explanation
Ans: An analysis of individual steps in the methodology that precisely controls the variables outlined in question 5 [maximum of two marks awarded].
Analysis and evaluation
Systematic errors are associated with flaws in the experimental design or instrumentation used while random errors are a result of uncontrolled variables.
A student performed a series of experiments to investigate how the reactivity of a metal affects the rate of formation of hydrogen gas when it is reacted with a strong acid. The method followed is outlined below.

Question:
Identify any systematic errors or random errors in the method designed by the student.
▶️Answer/Explanation
Ans: Systematic errors include the use of a beaker to measure hydrochloric acid; and the use of different surface areas of the metals (powder, ribbon and sheet); random errors include starting and stopping the stopwatch for the measurement of time; and fluctuations in the mass of the reaction mixture every 20 seconds.
Question:
Evaluate the method and identify any steps that require modification. Explain your reasoning.
▶️Answer/Explanation
Ans: The surface area for each of the metals must be consistent, for example, powdered zinc, magnesium and copper could be used; differences in the surface area of the metals alters the rate of the reaction and will prevent a valid conclusion being made upon analysis of the data; volume of hydrochloric acid should be measured with a measuring cylinder; differences in the volume of hydrochloric acid being used in each trial could affect the amount of hydrogen gas being produced in the reaction.
Question:
Explain how you could improve the method or extend the scope of the investigation.
▶️Answer/Explanation
Ans: Data logging equipment could be used to monitor the rate of change of mass; a gas pressure sensor could be used to monitor the rate of production of hydrogen gas; a wider variety of metals from the reactivity series could be investigated; more than one trial could be conducted to minimize the impact of random errors on the data.
Environmentally friendly anti-corrosion coatings
The following text is from the website of the United States Environmental Protection Agency. It outlines a project to investigate alternative anti-corrosion coatings.
Question:
Identify the problem for which the company Luna Innovations are aiming to develop solutions.
▶️Answer/Explanation
Ans: Corrosion or oxidation of metals.
Question:
Explain the risks involved in the current technology in use.
▶️Answer/Explanation
Ans: The current treatments – painting, priming and coating – are pollutants, toxic and possibly carcinogenic; they are therefore bad for the environment and potentially dangerous for the people applying them.
Question:
Green chemistry aims to reduce or eliminate the use or generation of hazardous substances. List the three advantages of the ISAM process that the company is developing and discuss the advantage of the green chemistry approach.
▶️Answer/Explanation
Ans: The ionic self–assembled monolayers (IASM) process has proven corrosion inhibition, does not contain or generate hazardous materials/waste; and has practical application methods; the advantage of the green chemistry approach to any industrial process is to reduce or eliminate the use or production of substances which are harmful to humans and the environment.
Question:
State four types of projects in which the process outlined could be used.
▶️Answer/Explanation
Ans: Building of ships, bridges, cars and aircrafts. Smaller industrial applications such as electric light fittings. Marks are awarded for four valid ideas.
Question:
Evaluate why this new coating process is an exciting innovation.
▶️Answer/Explanation
Ans: This new coating process is low cost; environmentally friendly; and easier and safer to work with. Marks are awarded for two valid responses.