1.1 Measurements in physics
Essential Idea:
Since 1948, the Système International d’Unités (SI) has been used as the preferred language of science and technology across the globe and reflects current best measurement practice
Understandings:
- Fundamental and derived SI units
- Scientific notation and metric multipliers
- Significant figures
- Orders of magnitude
- Estimation
Applications and Skills:
- Using SI units in the correct format for all required measurements, final answers to calculations and presentation of raw and processed data
- Using scientific notation and metric multipliers
- Quoting and comparing ratios, values and approximations to the nearest order of magnitude Estimating quantities to an appropriate number of significant figures
PHYSICAL WORLD
WHAT IS SCIENCE?
Purpose of science is to produce useful models of reality. Most scientific investigations use some form of the scientific method.
Science as defined above, is sometimes called pure science to differentiate it from applied science, which is the application of research to human needs. Fields of science are commonly divided into two major categories.
- Natural science : The science in which we study about the natural world. Natural science includes physics, chemistry, biology, etc.
- Social science : It is the systematic study of human behavior and society.
WHAT IS PHYSICS?
There are two main thrusts in physics :
- Unification : In physics, attempt is made to explain various physical phenomena in terms of just few concepts and laws. Attempts are being made to unify fundamental forces of nature in the pursuit of unification.
- Reductionism : Another attempt made in physics is to explain a macroscopic system in terms of its microscopic constituents. This pursuit is called reductionism.
Keep in memory
- Information received through the senses is called observation.
- An idea that may explain a number of observations is called hypothesis.
- A hypothesis that has been tested many times is called scientific theory.
- A scientific theory that has been tested and has always proved true is called scientific law.
SCOPE AND EXCITEMENT OF PHYSICS
Classical physics deals mainly with macroscopic phenomena consisting of the study of heat, light, electricity, magnetism, optics, acoustics, and mechanics. Since the turn of the 20th century, the study of quantum mechanics and relativity has become more important. Today, physics is often divided into branches such as nuclear physics, particle physics, quantum physics, theoretical physics, and solid-state physics. The study of the planets, stars, and their interactions is known as astrophysics, the physics of the Earth is called geophysics, and the study of the physical laws relating to living organisms is called biophysics.
Physics is exciting in many ways. Application and exploitation of physical laws to make useful devices is the most interesting and exciting part and requires great ingenuity and persistence of effort.
PHYSICS, TECHNOLOGY AND SOCIETY
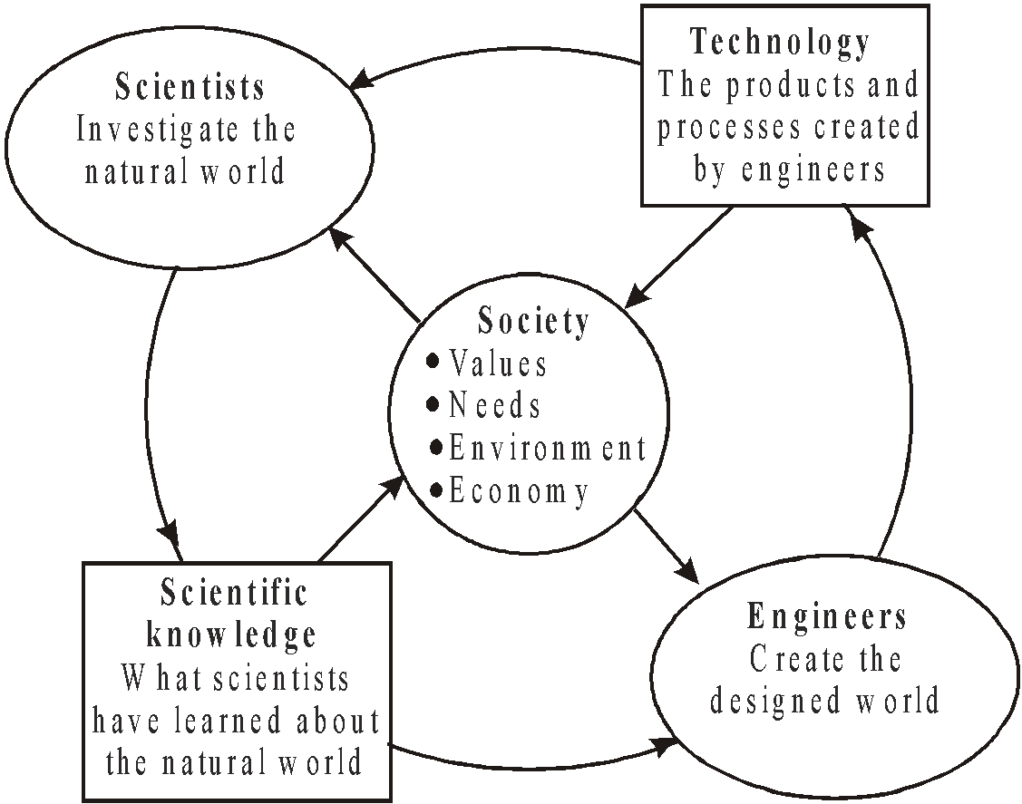
SCIENCE
- A body of knowledge
- Seeks to describe and understand the natural world and its physical properties
- Scientific knowledge can be used to make predictions
- Science uses a process–the scientific method–to generate knowledge
ENGINEERING
- Design under constraint
- Seeks solutions for societal problems and needs
- Aims to produce the best solution from given resources and constraints
- Engineering uses a process–the engineering design process–to produce solutions and technologies
TECHNOLOGY
- The body of knowledge, processes and artifacts that result from engineering
- Almost everything made by humans to solve a need is a technology
- Examples of technology include pencils, shoes, cell phones, and processes to treat water
LINK BETWEEN TECHNOLOGY AND PHYSICS
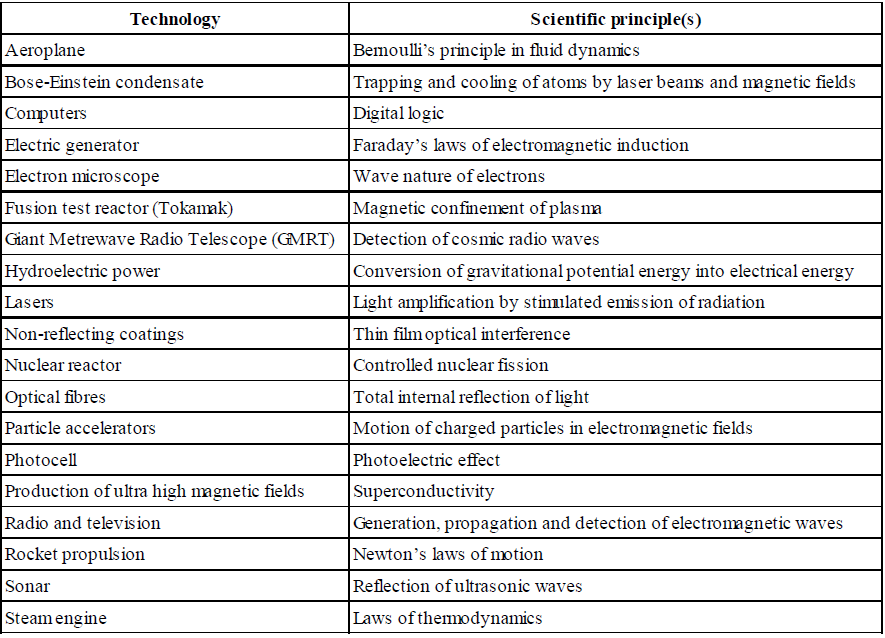
DOES IMAGINATION PLAY ANY ROLE IN PHYSICS?
HOW IS SCIENCE DIFFERENT FROM TECHNOLOGY?
FUNDAMENTAL FORCES IN NATURE
- Gravitational force
- Weak nuclear force
- Electromagnetic force
- Strong nuclear force
FUNDAMENTAL FORCES OF NATURE
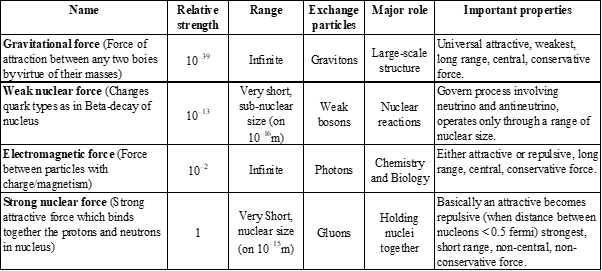
- Theories that postulate the unification of the strong, weak, and electromagnetic forces are called Grand Unified Theories (often known by the acronym GUTs).
- Theories that add gravity to the mix and try to unify all four fundamental forces into a single force are called Super Unified Theories.
- Theory that describes the unified electromagnetic and weak interactions is called the Standard Electroweak Theory, or sometimes just the Standard Model.
PROGRESS IN UNIFICATION OF DIFFERENT FORCES/DOMAINS IN NATURE
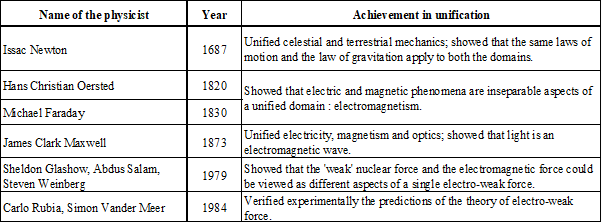
WHAT IS ELECTROMAGNETIC FORCE?
NATURE OF PHYSICAL LAWS
UNITS AND MEASUREMENTS
PHYSICAL QUANTITY
TYPES OF PHYSICAL QUANTITY
- Fundamental quantities : The physical quantities which do not depend upon other physical quantities are called fundamental or base physical quantities. e.g. mass, length, time temperature electric current, luminous intensity and amount of substance.
- Derived quantities : The physical quantities which depend on fundamental quantities are called derived quantities
e.g. speed, acceleration, force, etc.
UNIT
- It should be suitable in size (suitable to use)
- It should be accurately defined (so that everybody understands the unit in same way)
- It should be easily reproducible.
- It should not change with time.
- It should not change with change in physical conditions i.e., temperature, pressure, moisture etc.
- It should be universally acceptable.

FUNDAMENTAL (OR BASE) AND DERIVED UNITS
SYSTEM OF UNITS
DIFFERENT TYPES OF SYSTEM OF UNITS
C.G.S. (Centimetre – Gram – Second) system. (Gaussian system of units): In this system the unit of length is centimetre, mass is gram and time is second.
M.K.S (Metre – Kilogram – Second) system. This system is related to mechanics only. In this system the unit of length is metre, mass is kilogram and time is second.
S.I. (International system) units. (Introduced in 1971) Different countries use different set of units. To avoid complexity, by international agreement, seven physical quantities have been chosen as fundamental or base physical quantities and two as supplementary. These quantities are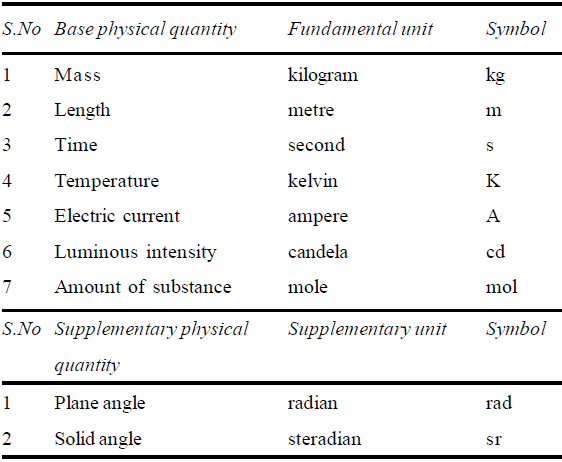
MERITS OF S.I. UNITS
- SI is a coherent system of units: This means that all derived units are obtained by multiplication and division without introducing any numerical factor.
- SI is a rational system of units: This is because it assigns only one unit to a particular physical quantity.
- SI is an absolute system of units: There is no gravitational unit in this system.
- SI system is applicable to all branches of science.
CONVENTIONS OF WRITING OF UNITS AND THEIR SYMBOLS
- Unit is never written with capital initial letter.
- For a unit named after scientist the symbol is a capital letter otherwise not.
- The unit or symbol is never written in plural form.
- Punctuations marks are not written after the symbol.
DEFINITIONS OF FUNDAMENTAL UNITS
- Metre : One metre is equal to 1650763.73 wavelength in vacuum of the radiation corresponding to transition between the levels 2p10 and 5d5 of the krypton – 86 atom
The distance travelled by light in vacuum in
- Kilogram : The mass of cylinder (of height and diameter 39 cm) made of Platinum-iridium alloy kept at International Bureau of weights and measures in paris is defined as 1kg.
- Second : It is the duration of 9,192,631,770 periods of radiation corresponding to the transition between the two hyperfine levels of the ground state of Caesium (133) atom.
- Ampere : It is the current which when flows through two infinitely long straight conductors of negligible cross-section placed at a distance of one metre in air or vacuum produces a force of 2 × 10–7 N/m between them.
- Candela : It is the luminous intensity in a perpendicular direction, of a surface of 1/600,000 square metre of a black body at the temperature of freezing platinum under a pressure of 1.013 × 105 N/m2.
- Kelvin : It is the 1/273.16 part of thermodynamic temperature of triple point of water.
- Mole : It is the amount of substance which contains as many elementary entities as there are in 0.012 kg of Carbon-12.
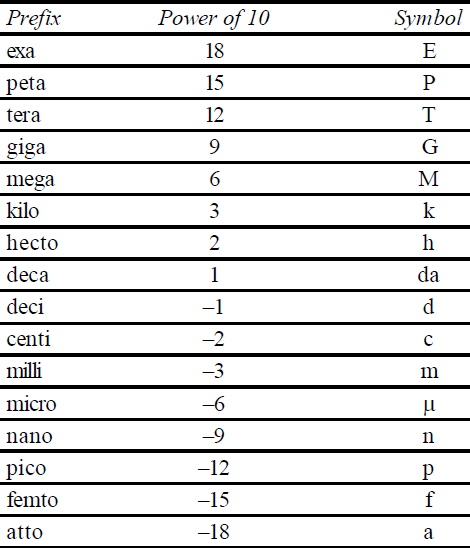
S.I. PREFIXES
SOME IMPORTANT PRACTICAL UNITS
- For large distance (macro-cosm)
1 A.U. = 1.496 × 1011m
1 ly = 9.46 × 1015m
1 parsec = 3.1 × 1016m
- For small distance (micro-cosm)
1 nanometre = 10–9m
1 angstorm = 10–10m
1 fermi = 10–15m
- For small area
1 barn = 10–28m2 - For heavy mass
1 ton = 1000kg
1 quintal = 100kg
1 slug = 14.57kg
1 C.S.L (chandrasekhar limit) = 1.4 times the mass of the sun - For small mass
1 amu = 1.67 x 10–27kg
1 pound = 453.6g = 0.4536 kg - For small time
1 shake = 10–8s - For large time
- For measuring pressure
DIMENSIONS
DIMENSIONAL FORMULA
DIMENSIONAL EQUATION
CLASSIFICATION OF PHYSICAL QUANTITIES (ON THE BASIS OF DIMENSIONS)

DIMENSIONAL FORMULA OF SOME IMPORTANT PHYSICAL QUANTITIES
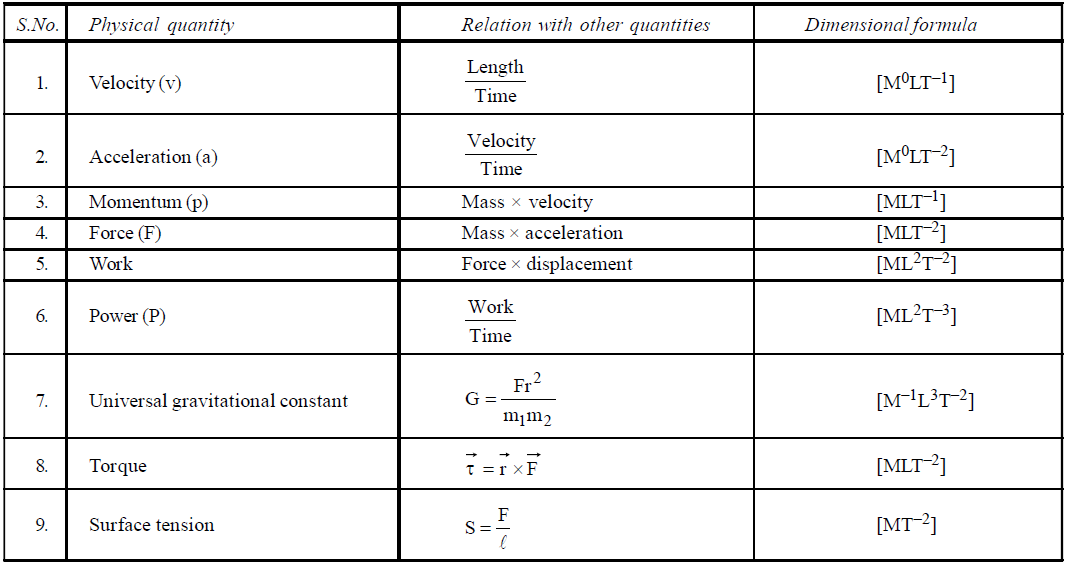
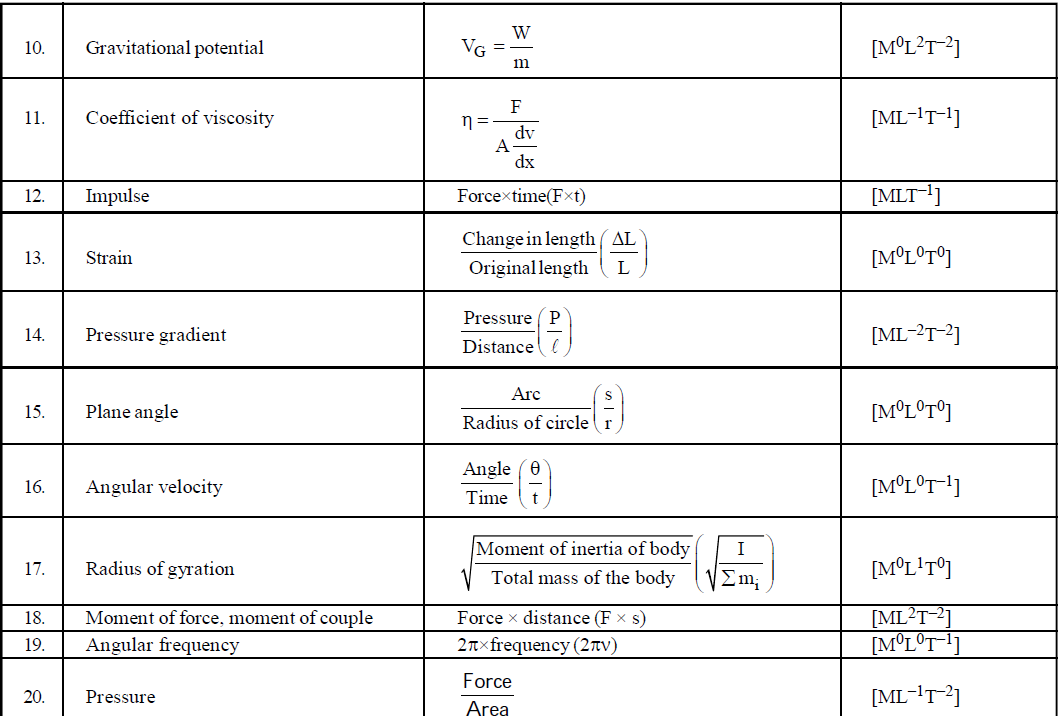
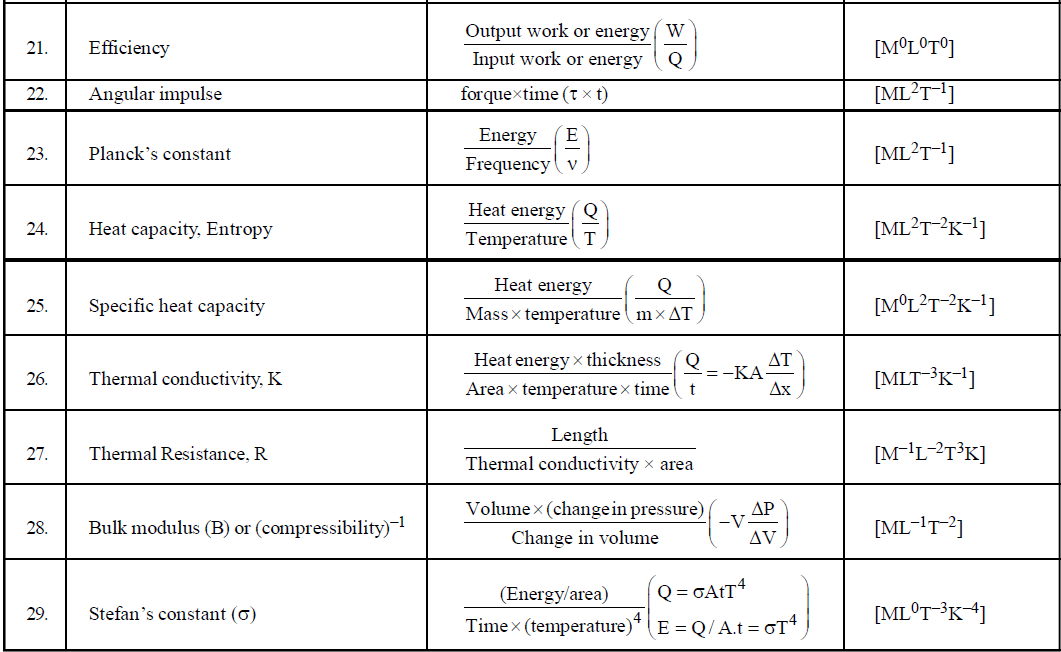
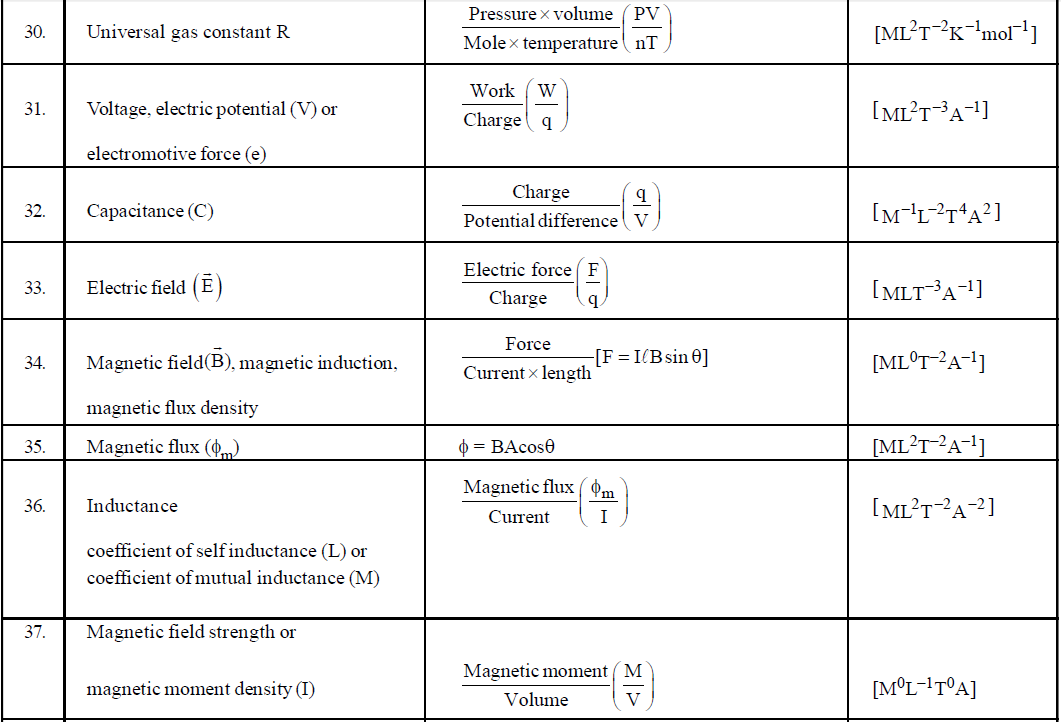
- To find dimensions of a typical physical quantity which is involved in a number of formulae, try to use that formula which is easiest for you. For example if you want to find the dimensional formula of magnetic induction then you can use the following formulae
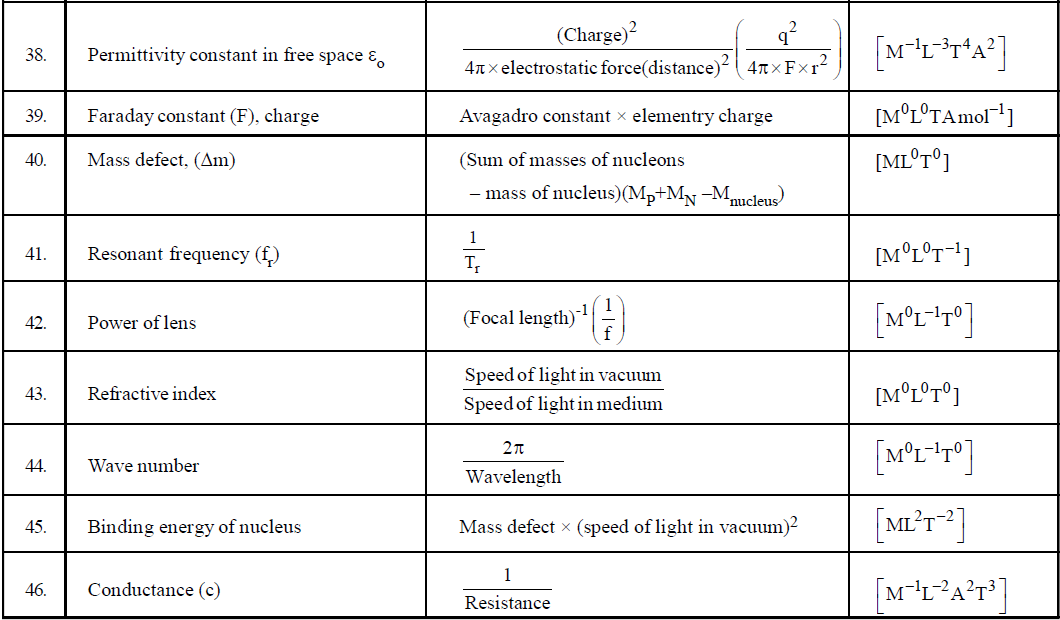
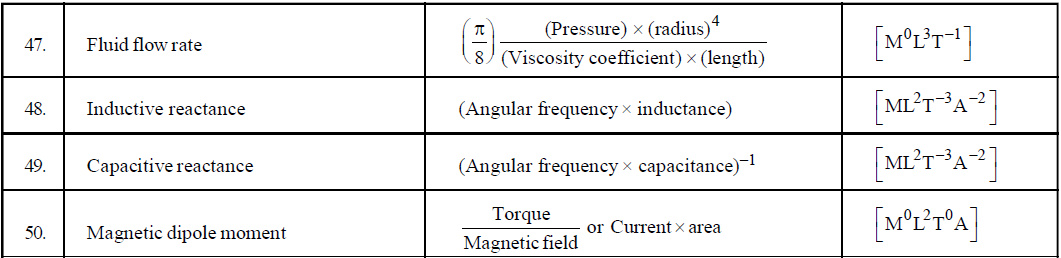
- If you have to find the dimensional formula of a combination of physical quantities, then instead of finding the dimensional formula of each, try to correlate the combination of physical quantities with a standard formula. For example, if you have to find the dimension of CV2, then try to use formula
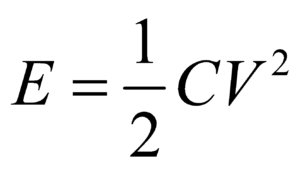 where E is energy of a capacitor.
where E is energy of a capacitor. 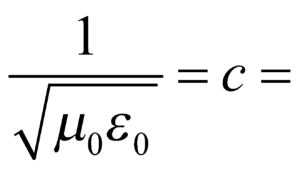 velocity of light in vacuum
velocity of light in vacuum- Dimensions of the following are same
- Dimensions of the following are same
- The dimension of RC =
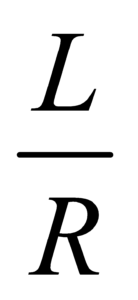 is same as that of time
is same as that of time - Dimensions of the following are same
- Dimensions of the following are same
- Dimensions of the following are same
- Dimensions of the following are same
- Dimensions of the following are same
KEEP IN MEMORY
- all trigonometric ratio is [M0L0T0]
- x in ex is [M0L0T0]
- ex is [M0L0T0]
- x in log x is [M0L0T0]
- log x is [M0L0T0]
DIMENSIONAL ANALYSIS AND ITS APPLICATIONS
USES OF DIMENSIONS
- Conversion of one system of unit into another
- Checking the accuracy of various formulae
- Derivation of formula
LIMITATIONS OF DIMENSIONAL ANALYSIS
- No information about the dimensionless constant is obtained during dimensional analysis
- Formula cannot be found if a physical quantity is dependent on more than three physical quantities.
- Formula containing trigonometrical /exponential function cannot be found.
- If an equation is dimensionally correct it may or may not be absolutely correct.
SIGNIFICANT FIGURES
RULES TO DETERMINE THE NUMBERS OF SIGNIFICANT FIGURES
- All non-zero digits are significant. 235.75 has five significant figures.
- All zeroes between two non-zero digits are significant. 2016.008 has seven significant figures.
- All zeroes occurring between the decimal point and the non-zero digits are not significant provided there is only a zero to left of the decimal point. 0.00652 has three significant figures.
- All zeroes written to the right of a non-zero digit in a number written without a decimal point are not significant. This rule does not work if zero is a result of measurement. 54000 has two significant figures whereas 54000m has five significant figures.
- All zeroes occurring to the right of a non-zero digit in a number written with a decimal point are significant. 32.2000 has six significant figures.
- When a number is written in the exponential form, the exponential term does not contribute towards the significant figures. 2.465 × 105 has four significant figures.
KEEP IN MEMORY
- The significant figures depend upon the least count of the instrument.
- The number of significant figure does not depend on the units chosen.
ROUNDING OFF
- If digit to be dropped is less than 5 then preceding digit should be left unchanged.
- If digit to be dropped is more than 5 then one should raise preceding digit by one.
- If the digit to be dropped is 5 followed by a digit other than zero then the preceding digit is increased by one.
- If the digit to be dropped is 5 then the preceding digit is not changed if it is even.
- If digit to be dropped is 5 then the preceding digit is increased by one if it is odd.
ARITHMETICAL OPERATIONS WITH SIGNIFICANT FIGURES AND ROUNDING OFF
- For addition or subtraction, write the numbers one below the other with all the decimal points in one line. Now locate the first column from the left that has a doubtful digit. All digits right to this column are dropped from all the numbers and rounding is done to this column. Addition subtraction is then done.
Step-2 :- 23.6 + 8.7=32.3
- In multiplication and division of two or more quantities, the number of significant digits in the answer is equal to the number of significant digits in the quantity, which has minimum number of significant digits.
ACCURACY, PRECISION OF INSTRUMENTS AND ERRORS IN MEASUREMENTS
The following diagrams illustrate the meaning of terms accuracy and precision
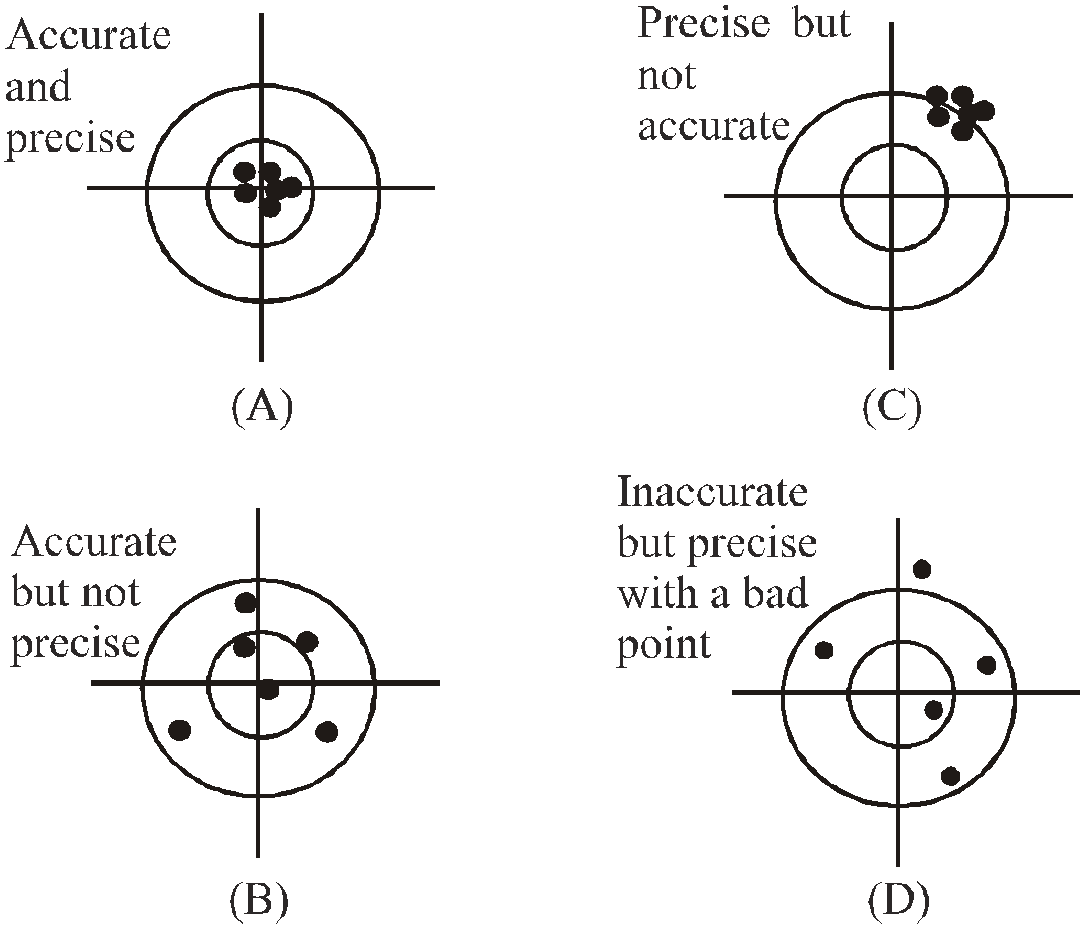
- Accuracy depends on the least count of the instrument used for measurement.
- In the addition and subtraction operation, the result contains the minimum number of decimal places of the figures being used
- In the multiplication and division operation, the result contains the minimum number of significant figures.
- Least count (L.C.) of vernier callipers = one MSD – one VSD
- Least count of screw gauge (or spherometer)
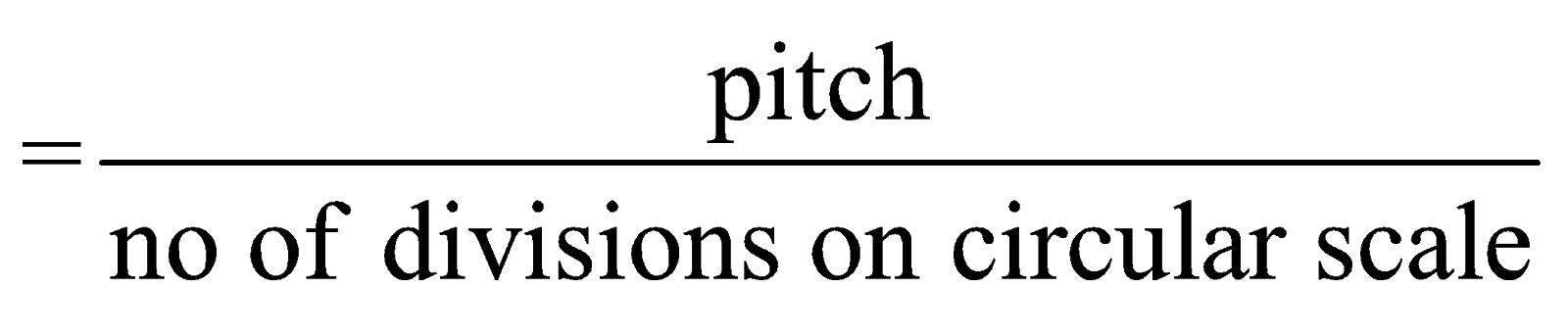
where pitch is the ratio of number of divisions moved on linear scale and number of rotations given to circular scale. - Pure number or unmeasured value do not have significant numbers
- Change in the position of decimal does not change the number of significant figures. Similarly the change in the units of measured value does not change the significant figures.
COMMON ERRORS IN MEASUREMENTS
EXPERIMENTAL ERRORS
- by the lack of perfection of observer
- if the measuring instrument is not perfectly sensitive.
- measuring instrument having a zero error.
- an instrument being incorrectly calibrated (such as slow- running-stop clock)
- the observer persistently carrying out a mistimed action (e.g., in starting and stopping a clock)
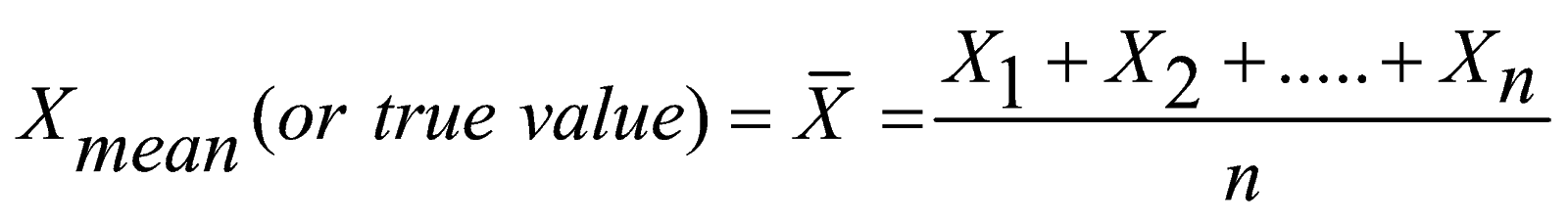
METHODS OF EXPRESSING ERROR
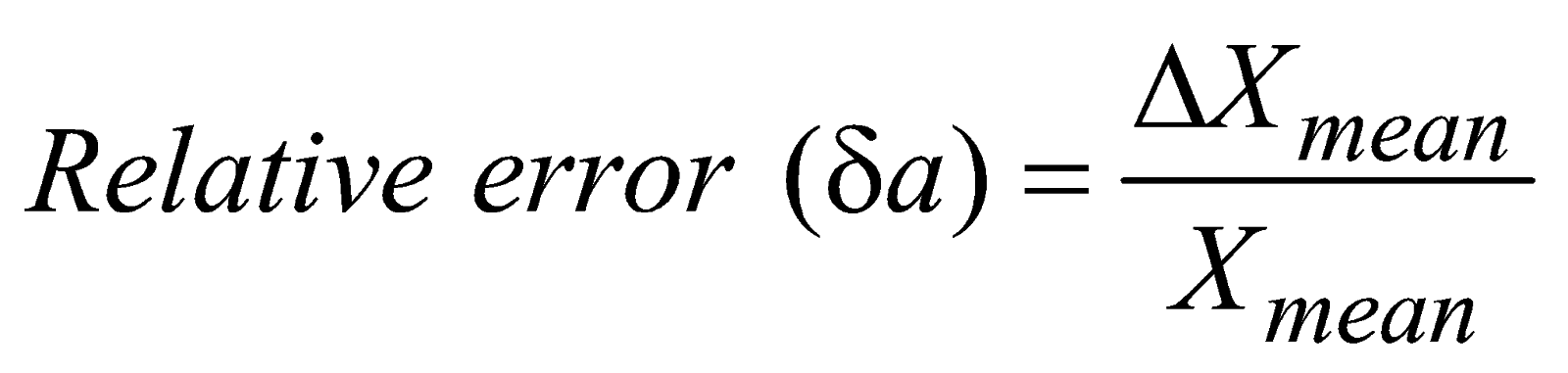
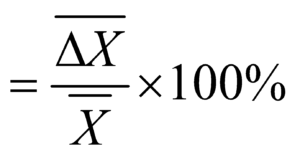
TO FIND THE MAXIMUM ERROR IN COMPOUND QUANTITIES
We have to find the sum or difference of two values given as (a ± Δa) and (b ± Δb), we do it as follows
PRODUCT AND QUOTIENT
We add the fractional or percentage errors in case of finding product or quotient.
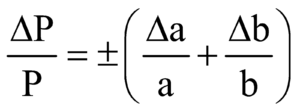
If x = an then

- More the accuracy, smaller is the error.
- Absolute error |ΔX| is always positive.
- |ΔX| has the same dimensions as that of X.
- If the least count of measuring instrument is not given and the measured value is given the least error in the measurement can be found by taking the last digit to be 1 and rest digit to be zero. For e.g. if the measured value of mass m = 2.03 kg then
.
- If a number of physical quantities are involved in an expression then the one with higher power contributes more in errors and therefore should be measured more accurately.
- Relative error is a dimensionless quantity.
- We are always interested in calculating the maximum possible error.
The realm of physics
1.1.1 State and compare quantities to the nearest order of magnitude.
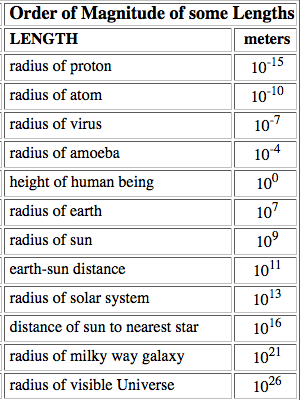
Throughout the study of physics we deal with a wide range of magnitudes. We will use minuscule values such as the mass of an electron and huge ones such as the mass of the (observable) universe. In order to easily understand the magnitude of these quantities we need a way to express them in a simple form, to do this, we simply write them to the nearest power of ten (rounding up or down as appropriate).
That is, instead of writing a number such as 1000, we write 103 .
The use of orders of magnitude is generally just to get an idea of the scale and differences in scale of values. It is not an accurate representation of a value. For example, if we take 400, it’s order of magnitude is 102 , which when we calculate it gives 10 x 10 = 100. This is four times less than the actual value, but that does not matter. The point of orders of magnitude is to get a sense of the scale of the number, in this case we know the number is within the 100s.
1.1.2 State the ranges of magnitude of distances, masses and times that occur in the universe, from smallest to greatest.
Distances:
- sub-nuclear particles: 10-15 m
- extent of the visible universe: 10+25 m
Masses:
- mass of electron: 10-30 kg
- mass of universe: 10+50 kg
Times:
- passage of light across a nucleus: 10-23 s
- age of the universe : 10+18 s
1.1.3 State ratios of quantities as differences of orders of magnitude.
Using orders of magnitude makes it easy to compare quantities, for example, if we want to compare the size of an an atom (10-10 m) to the size of a single proton (10-15 m) we would take the difference between them to obtain the ratio. Here, the difference is of magnitude 105meaning that an atom is 105 or 100000 times bigger than a proton.
1.1.4 Estimate approximate values of everyday quantities to one or two significant figures and/or to the nearest order of magnitude.
Significant figures
To express a value to a certain amount of significant figures means to arrange the value in a way that it contains only a certain amount of digits which contribute to its precision.
For example, if we were asked to state the value of an equation to three significant figures and we found the result of that value to be 2.5423, we would state it as 2.54.
Note that 2.54 is accurate to three significant figures as we count both the digits before and after the point.
The amount of significant figures includes all digits except:
- leading and trailing zeros (such as 0.0024 (2 sig. figures) and 24000 (2 sig. figures)) which serve only as placeholders to indicate the scale of the number.
- extra “artificial” digits produced when calculating to a greater accuracy than that of the original data, or measurements reported to a greater precision than the equipment used to obtain them supports.
Rules for identifying significant figures:
- All non-zero digits are considered significant (such as 14 (2 sig. figures) and 12.34 (4 sig. figures)).
- Zeros placed in between two non-zero digits (such as 104 (3 sig. figures) and 1004 (4 sig. figures))
- Trailing zeros in a number containing a decimal point are significant (such as 2.3400 (5 sig. figures) note that a number 0.00023400 also has 5 sig. figures as the leading zeros are not significant).
Note that a number such as 0.230 and 0.23 are technically the same number, but, the former (0.230) contains three significant figures, which states that it is accurate to three significant figures. On the other hand, the latter (0.23) could represent a number such as 2.31 accurate to only two significant figures. The use of trailing zeros after a decimal point as significant figures is is simply to state that the number is accurate to that degree.
Another thing to note is that some numbers with no decimal point but ending in trailing zeros can cause some confusion. For example, the number 200, this number contains one significant figure (the digit 2). However, this could be a number that is represented to three significant figures which just happens to end with trailing zeros.
Typically these confusions can be resolved by taking the number in context and if that does not help, one can simply state the degree of significance (for example “200 (2 s.f.)” , means that the two first digits are accurate and the second trailing zero is just a place holder.
Expressing significant figures as orders of magnitude:
To represent a number using only the significant digits can easily be done by expressing it’s order of magnitude. This removes all leading and trailing zeros which are not significant.
For example:
0.00034 contains two significant figures (34) and fours leading zeros in order to show the magnitude. This can be represented so that it is easier to read as such: 3.4 x 10-4 .
Note that we simply removed the leading zeros and multiplied the number we got by 10 to the power of negative the amount of leading zeros (in this case 4). The negative sign in the power shows that the zeros are leading.
A number such as 34000 (2 s.f.) would be represented as 34 x 103 .
Again, we simply take out the trailing zeros, and multiply the number by 10 to the power of the number of zeros (3 in this case).
There are a couple of cases in which you need to be careful:
- A number such as 0.003400 would be represented as 3.400 x 10-3 . Remember, trailing zeros after a decimal point are significant.
- 56000 (3 s.f.) would be represented as 560 x 102 . This is because it is stated that the number is accurate the 3 significant zeros, therefor the first trailing zero is significant and must be included. Note that this can be used as another way to express a value such as 56000 to three significant figures (as opposed to writing “56000 (3 s.f.)”).
Rounding
When working with significant figures you will often have to round numbers in order to express them to the appropriate amount of significant figures.
For example:
State 2.342 to three significant figures: would be written 2.34.
When representing the number 2.342 to three significant figures we rounded it down to 2.34. This means that when we removed the excess digit, it was not high enough to affect the last digit that we kept.
Whether to round up or down is a simple decision:
If the first digit in the excess being cut off is lower than 5 we do not change the last digit which we are keeping. If the first digit in the excess which is being cut off is 5 or higher we increment the last digit that we are keeping (and the rest of the number if required).
For example:
State 5.396 to three significant figures: would be written 5.40. This is because we remove the last digit (6) in order to have three significant digits. However, 6 is large enough for it to affect the digit before it, therefor we increment the last digit of the number we are keeping by 1. Note that the last digit of the number that we are keeping is 9, therefor incrementing it by 1 gives 10. Thus we also need to increment the second to last digit by 1. This gives 5.40 which is accurate to three significant figures.
You might think that if a number such as 5.4349 were to be rounded to 3 s.f. it would give 5.44 as the last digit is 9 which is large enough to affect the previous digit which would then become 5. Now that 5 would be large enough to affect the last digit of the number we are keeping which would become 4 (thus 5.44). However, this is not the case, when rounding, we only look at the digit immediately after the one we are rounding to, whether or not that digit would be affected by the one after it is not taken into account. Therefor, the correct result of this question would be 5.43.
1. Estimate the number of seconds in a human “lifetime”.
Answer: 70yrs = 2.2 * 10^9 sec, 100yrs = 3.1 * 10^9 sec, 50yrs = 1.6 * 10^9 sec, therefore the estimate of the order of magnitude is 10^9 sec.
|
|
1. Estimate the ratio of
a) the mass of an electron to the mass of a human
b) the radius of earth to the size of the universe
Answers:
1. a) compare 10^-30 and 10^2, 10^32 is the difference hence 1:10^32 b) compare 10^7 and 10^26, 10^19 is the difference hence 1:10^19
Significant figures are the individual digits in a numerical value. For example, the number 20 has two significant figures. One important rule regarding significant figures is that when there are zeroes preceding the number (ie. 0.020), they are not counted, hence the value 0.020 would still have two significant figures. Zeroes after the number (ie. 20.00) or between values (ie. 201) are always counted.
In order to express significant figures as orders of magnitude, one should take all of the numbers following the amount of significant figures given and convert them to a power of 10. For example, the number 0.0030 would be 3.0 × 10^-4; the zero after 3 remains because it was significant in the original value. Sometimes a significant figure amount will be given to you, for example, “56,000 (3 s.f.)” would convert to 560*10^2 because 560 has three significant figures and that was the requested amount in the original question.
Sometimes numbers need to be rounded in order to fit a significant figure amount. If the amount of significant figures required is two and your answer is “56,000”, you should rewrite it as “5.6 × 10^4”. Similarly, if your answer is “0.0560823” and you require two significant figures, you should round it down to “0.056” or rewrite it as “5.6 × 10^-2”.


