| Biomolecules Refresher Course |
| Biomolecules Concepts Files |
| Biomolecules Master Files |
| Biomolecules Notes 1 |
| Biomolecules Note 2 |
Biomolecules
Table of Content
- Define Biomolecule
- Proteins
- Protein Structure
- Nucleic Acids
- Structure of Double Stranded DNA
- RNA
- Carbohydrates
- Lipids
What are Biomolecules?
Biomolecule is an organic molecule that is produced by living organism. They act as building block of life and perform important functions in living organisms. They are primarily composed of carbon, hydrogen, nitrogen, oxygen, phosphorous and Sulphur. The four common biomolecules are: Proteins, Nucleic Acid, Carbohydrates, and Lipids.
Proteins
Any of a class of nitrogenous organic compounds which have large molecules composed of one or more long chains of amino acids and are an essential part of all living organisms.
The building blocks of proteins are known as Amino Acids. There are 22 naturally occurring amino acids found in nature. Amino Acids are made up of Carbon, Hydrogen, Oxygen and Nitrogen. So, single amino acid is made up of Amino Group, Carboxyl Group, Hydrogen Atom and distinctive side chain, all bonded to alpha-carbon.

Fig.1. Structure of Amino Acid
When Amino Acids are dissolved in water, it exists in solution as the Dipolar Ion or Zwitterion. They can either acts as proton donor or proton acceptor.
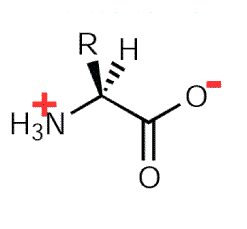
Fig. 2. Zwitterion
All Amino Acids are optically active, that is, they can rotate the plane of polarized light. Optically active molecules contain chiral carbon except glycine. A tetrahedral carbon atom with four different constituents are said to be chiral.
Peptide is a compound consisting of two or more amino acids. When two amino acids are linked together via peptide bond, they are said to form a dipeptide. Three Amino Acids joined to form tripeptide etc. Peptide chains of 12 to 20 amino acids form oligopeptide. When many amino acids are joined, they form polypeptide. The first amino acid is known as N Terminal or Amino Terminal and Last Amino Acid is said to be C terminal or carboxyl terminal.
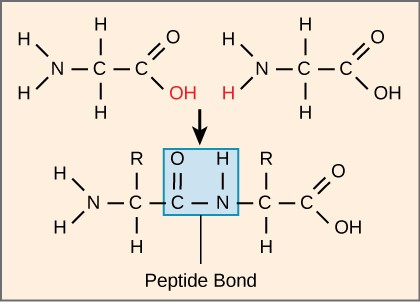
Fig. 3. Formation of peptide bond
Protein Structure
Proteins have four levels of protein organization. They are as follows:
Primary Structure of the protein is its sequence of amino acids. Amino Acids are joined by a peptide bond.
Secondary Structures are higher level of protein organization which includes- alpha helix and beta sheets. Both of them are stabilized by hydrogen bonds between carbonyl and N-H groups in a polypeptide backbone.
Alpha Helix is a rigid, rod like structure that forms when a polypeptide chain twists into a helical conformation.
Beta Pleated Sheets are formed when two or more polypeptide chain segment line up side by side. Each individual segment is known as Beta Strand.
Tertiary Structures refer to the three-dimensional conformations that a protein assumes as a consequence of the interactions between the side chains in their primary structure. They are stabilized by hydrophobic interactions, electrostatic interactions, hydrogen bonds, van der waal forces and covalent bonds.
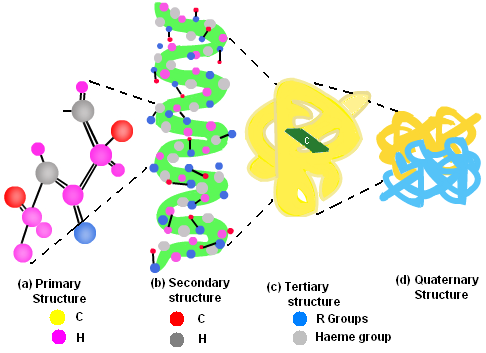
Fig. 4. Levels of protein organization
Quaternary Structures are composed of two or more polypeptide chains. Polypeptides are held together via hydrophobic interactions, electrostatic interactions, and hydrogen bonds etc. For Example: Hemoglobin.
Fibrous and globular proteins
Fibrous are long, rod shaped molecules which are insoluble in water. They are generally protective and structural in nature. Globular proteins are compact spherical molecules that are usually water soluble.
Nucleic Acids
Nucleic Acid was first discovered by Friedrich Miescher from the nuclei of pus cells. There two types of Nucleic Acids- Deoxyribonucleic Acids and Ribonucleic Acids.
The monomeric unit of nucleic acid is known as Nucleotides. Nucleotide is made up of three components: A Nitrogenous Base, A Five-Carbon Sugar and Phosphoric Acid.
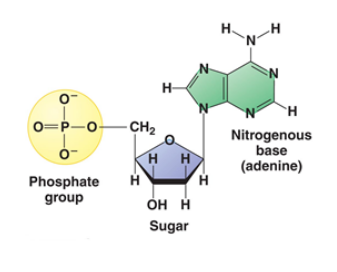
Fig. 5. Structure of Nucleotide
Nitrogenous bases are heterocyclic, aromatic molecules. There are basically two types of Nucleic Acids: Purines and Pyrimidines.
Purines are of two types – Adenine and Guanine whereas pyrimidines can be thymine, cytosine, or uracil.
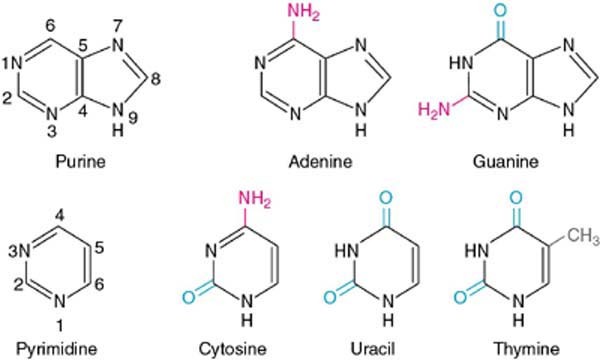
Fig. 6. Structure of purines and pyrimidines
The 5- carbon sugar found in DNA is deoxyribose and for RNA is ribose. Nucleotide without phosphate is known as nucleoside.
Structure of Double Stranded DNA
DNA are of different types such as:
B DNA
- Two long polynucleotide strands are coiled around the axis.
- Strands are antiparallel to each other.
- Guanine base pairs with cytosine and adenine base pairs with thymine.
- Guanine forms 3 hydrogen bonds with cytosine whereas adenine forms two hydrogen bonds with thymine.
Z DNA
- They are thinner than B DNA.
- They have alternating purine and pyrimidine bases.
- They are stabilized by high salt concentration.
Denaturation of DNA
When DNA is subjected to varying pH, temperature etc, two DNA strands separate out. That is the DNA is said to be denatured. If the denaturant such as temperature, pH is removed, the DNA regains its original or native structure. This is known as Renaturation.
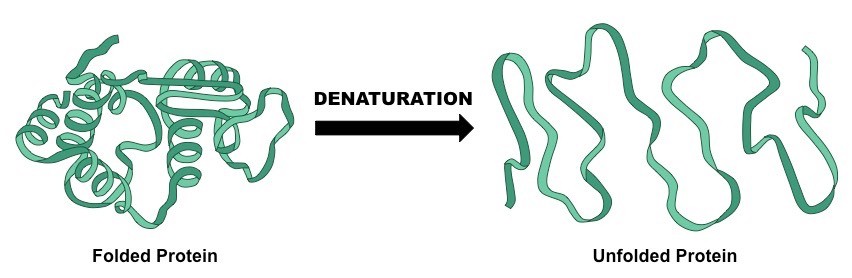
Fig. 7. Process of denaturation
RNA
RNA known as Ribonucleic Acid. It usually exists as single strand. RNA exists in cells in various forms as explained below-
Messenger RNA carries genetic information from DNA in the form of codons (three nucleotides forms a codon), which codes for amino acids or protein.
Transfer RNA plays an important role during protein synthesis. They act as an interface between nucleic acid language and protein language. They are fund in the cytosol of the living cell.
Ribosomal RNA is a component of ribosomes. They have important roles during protein synthesis in eukaryotes and prokaryotes.
Carbohydrates
They are defined as the polyhydroxy aldehydes or polyhydroxy ketones. They consist of hydrogen, carbon, and oxygen. The simplest carbohydrates are known as Monosaccharides, such as glucose. Two monosaccharides joined to form disaccharides. Polymers of 2 to 10 units of monosaccharides is known as Oligosaccharides. When hundreds to thousands of monosaccharides are joined, they are known as Polysaccharides.
Monosaccharides with aldehyde group is known as Aldoses. And monosaccharides with ketone group is known as Ketoses. Trioses are the simplest monosaccharides.
Some common saccharides
Starch is the storage form of energy is plants. It is a branched polysaccharide made up of glucose units. It is mixture of two different polymers amylose and amylopectin. Amylose is the unbranched polymer of glucose units whereas amylopectin is the branched polymer of glucose units.
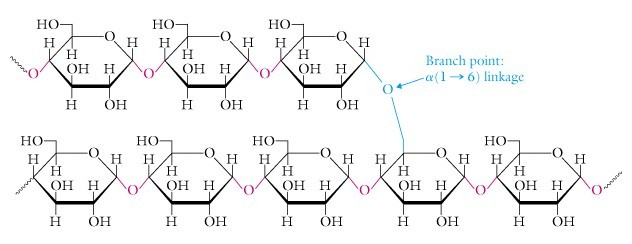
Fig. 8. Structure of starch
Glycogen is the storage form of energy in animals. It is stored in muscle and liver. It is also a highly-branched polymer made up of amylose and amylopectin.
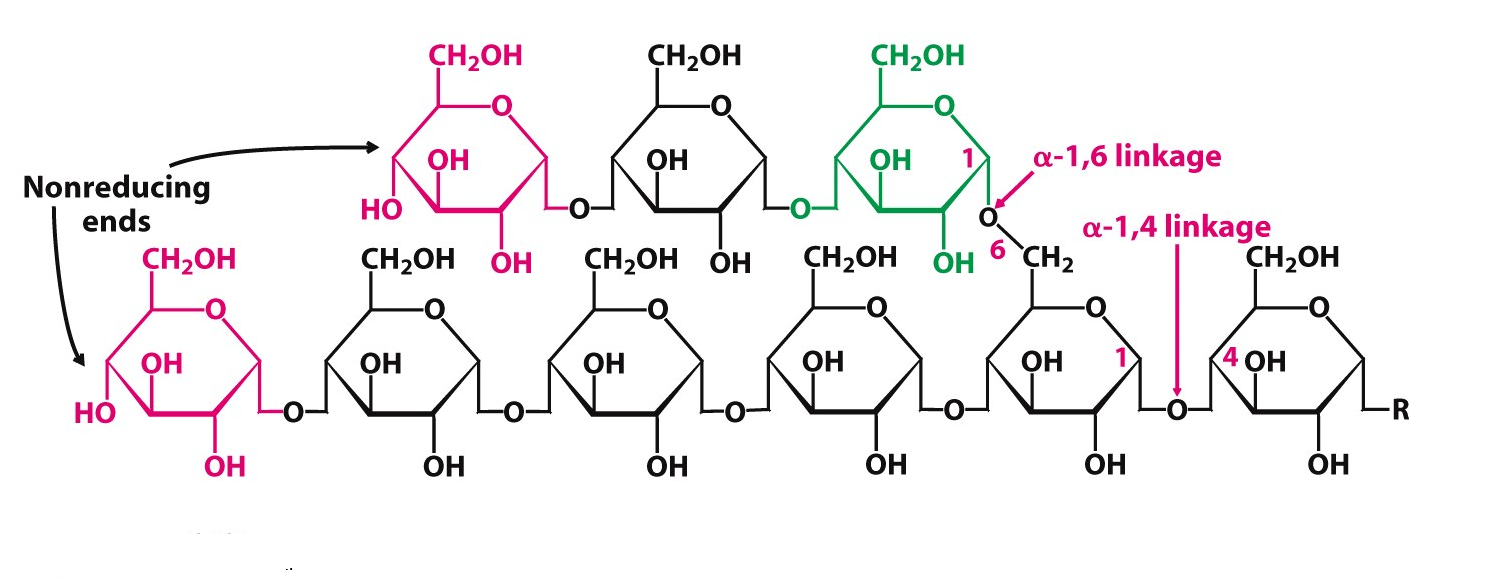
Fig. 9. Structure of glycogen
Cellulose is an unbranched polymer of glucose units. It is a structural polysaccharide of plant cells. It is the most abundant organic molecule in the biosphere. It is found in plant cells and provide strength and rigidity to the cell.
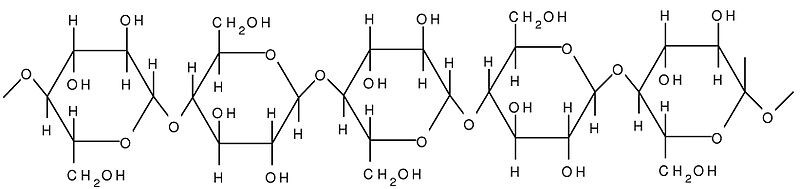
Fig. 10. Structure of cellulose
Chitin is a linear polysaccharide composed of N-acetyl-D-glucosamine residues. It is found in the exoskeleton of insects and crustaceans.
Reducing and Non-Reducing Sugar
Sugars capable of reducing ferric or cupric ion are called reducing sugar. All monosaccharides are reducing sugars.
Non-reducing sugars are not capable of reducing ferric or cupric ion. For Example: Sucrose is a Non-Reducing Sugar.
Lipids
They are insoluble or poorly insoluble in water. They are soluble in non-polar solvents such as ether, chloroform or benzene.
Biological functions of Lipids
- They serve as storage form of energy.
- They are major component of membranes.
- They are protective in function such as in bacteria, plants, insects, and vertebrates.
Fatty Acids
They are the simplest form of lipids. They are composed of long chains of hydrocarbons with one carboxyl group. The alkyl chain may be saturated or unsaturated. Unsaturated fatty acids may contain one or more double bonds. They have both polar and non-polar ends. Mammals are unable to synthesize some fatty acids such as linoleate. Such fatty acids must be obtained from the diet, they are known as Essential Fatty Acids. Some Fatty Acids can be synthesized endogenously, they are known as Non-Essential Fatty Acids.
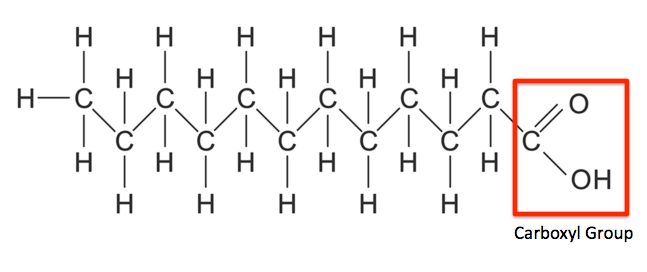
Fig. 11. Structure of Fatty Acids
Triacylglycerol also known as Triglycerides are esters of fatty acids and glycerol. They are non-polar, hydrophobic in nature.