| Mineral Nutrition Refresher Course |
| Mineral Nutrition Concepts Files |
| Mineral Nutrition Master Files |
| Mineral Nutrition Revision Note |
Mineral Nutrition
Table of Content
- Mineral Nutrition in Plants
- Methods to study the Mineral requirement of Plants
- Deficiency Symptoms of Essential Elements
- Metabolism of Nitrogen
Mineral Nutrition in Plants
Every living organisms need carbohydrates, proteins, fats, water, and minerals to live. Similarly, plants need nutrients for growth and development.
Methods to study the Mineral requirement of Plants
- Hydroponics is a technique in which plants are grown in nutrient solution instead of soil. This technique is used at the time of commercial production of vegetables. It is also used to study the mineral deficiency diseases in plants.
- Aeroponics is the technique in which nutrients are sprayed suspended in the air.
Essential Mineral Elements
Different plants have different mineral requirements. There is criterion for the essentiality of an element. It includes:
- The element must be necessary for normal growth and reproduction. In the absence of that element the plants will not be able to complete its life cycle.
- The requirement of the element must be specific and it should not be replaceable by any another element.
- The element must be directly involved in the metabolism of the plant.
The mineral elements needed by the plant is divided into- macronutrients and micronutrients.
Macronutrients and Micronutrients
Macronutrients
These elements are needed by the plants in large quantities. It includes carbon, hydrogen, oxygen, nitrogen, phosphorous, sulphur, potassium, calcium and magnesium. Out of these, carbon, hydrogen and oxygen are mainly obtained from CO2 and H2O, while the others are absorbed from the soil as Mineral Nutrition.
Micronutrients
These are the nutrients that are needed by the plants in small quantity. It includes iron, manganese, copper, molybdenum, zinc, boron, chlorine and nickel.
Essential elements are grouped in different categories based on their diverse functions such as-
- Essential elements such as carbon, hydrogen, oxygen and nitrogen serves as components of various biomolecules such as amino acids, lipids and hence structural elements of cells
- Essential elements are major components of energy-related chemical compounds such as magnesium ion in chlorophyll and phosphorous in ATP.
- Essential elements are also activator and inhibitor of certain enzymes such as Mg2+ is an activator for both ribulose bisphosphate carboxylase oxygenase and phosphoenol pyruvate carboxylase, both of which are directly involved in carbon fixation. Zn2+ is an activator of alcohol dehydrogenase and Mo is essential component for nitrogenase activity during nitrogen metabolism.
- Some essential elements are involved in changing osmotic potential of a cell. Potassium plays an important role in the opening and closing of stomata.
Role of Macronutrients and Micronutrients
The role of different macro and micronutrients are given below-
Nitrogen
- Essential element required by the plants in large quantities.
- It is absorbed by the plants in the form of nitrate ions (NO3)– and some plants also absorb in the form of nitrite ions(NO2)– or ammonium ions(NH4)+.
- Needed by the plants for actively growing tissues such as meristematic tissues.
- It is a necessary component of vitamins.
- Directly involved in photosynthesis.
Phosphorous
- It is absorbed by the plants in the form of phosphate ions (PO4)3-.
- It is major component of cell membranes, proteins, nucleic acids and nucleotides.
- Promotes root formation and growth.
- Required for seed formation.
- Involved in energy storage and transfer.
Potassium
- It is absorbed by the plants in the form of potassium ions.
- It is abundant in actively growing tissues such as meristematic tissues, buds, leaves and root tips.
- It is required component for maintaining osmotic potential in a cell which is responsible for opening and closing of the stomata.
- Increases the rate of photosynthesis.
- Essential for protein synthesis.
Calcium
- It is absorbed by the plants in the form of calcium ions(Ca2+).
- Required for continuous cell division and growth.
- Involved in nitrogen metabolism.
- It is present in the middle lamella in the form of calcium pectate.
- It is also required for the formation of mitotic spindle.
- It is also required for the activation of the certain enzymes.
Magnesium
- It is absorbed by the plants in the form of magnesium ions(Mg2+).
- It is required for the activation of the enzymes involved in respiration, photosynthesis.
- It is involved in the synthesis of RNA and DNA.
- Key component of chlorophyll.
- It also maintains ribosome structure.
Sulphur
- Absorbed in the form of sulphate ions(SO4)2-.
- Integral component of amino acids such as methionine.
- Component of several coenzymes vitamins such as thiamine, biotin, and ferrodoxin.
- Helps in seed production.
- Required for chlorophyll formation.
Iron
- Plants absorb iron mainly in the form of ferric ions(Fe3+).
- Involves in cell division and growth.
- Involves in electron transfer during various metabolic reactions.
- It also activates an enzyme catalase required for chlorophyll formation.
- Serves as oxygen carrier.
Manganese
- It is absorbed in the form of manganese ions(Mn2+).
- It is involved in photolysis of water during non-cyclic photophosphorylation.
- One of the component of nitrogenase enzyme required for nitrogen metabolism.
Zinc
- It is absorbed in the form of zinc ions(Zn2+).
- Required for chlorophyll formation.
- Involved in activation of various enzymes such as carboxylases.
- Needed for carbohydrate formation.
Copper
- It is absorbed by the plants in the form of cupric ions (Cu2+).
- It is involved in redox reactions during metabolic reactions.
- Works during photosynthesis and reproductive stages of the plants.
- Involves in flavor of fruits and vegetables.
- Increase the sugar content of the plants.
Boron
- It is absorbed by the plants in the form of BO33− or B4O72-.
- Involved in the uptake of calcium by the plants.
- Functions in pollen tube formation.
- Carbohydrate translocation in plants.
- Involved in cell differentiation.
Molybdenum
- Plants obtain it in the form of molybdate ions (MoO2)2-.
- Major component of nitrogenase enzyme. It forms a catalytic site of the enzyme along with iron.
- It is also a component of nitrate reductase involved in nitrogen assimilation.
- Participates in nodule formation.
Chlorine
- Absorbed in the form of chloride ions (Cl1-).
- Maintains the osmotic potential of the cell.
- It is involved in photolysis of water for evolution of oxygen.
Deficiency Symptoms of Essential Elements
Element | Deficiency Symptoms | Images |
Nitrogen | Yellowing of leaves is the important symptom associated with nitrogen deficiency |
Fig.1. |
Phosphorous | Leaf tips appear burnt and yellow |
Fig.2. |
Potassium | Interveinal chlorosis, older leaves wilt |
Fig.3. |
Calcium | Deficiency causes blossom end rot |
Fig. 4. |
Magnesium | Older leaves appear yellow, that is, chlorosis of leaf due to degradation chlorophyll of the leaves |
Fig.5. |
Sulphur | Young leaves appear yellow first followed by older leaves |
Fig.6. |
Iron | Chlorosis of young leaves, dieback disease is the characteristic disease of deficiency of iron in plants, stunted growth |
Fig.7. |
Manganese | Yellowing in between the veins of the leaves, reduce plant parts with dead spots |
Fig.8. |
Zinc | Rosette formation, yellowing between the veins, stunted growth of the plant |
Fig.9. |
Copper | Weakening of cell wall strength, dieback of stems and twigs |
Fig.10. |
Boron | Affects the reproductive as well as vegetative growth of the plants, death of meristem |
Fig.11. |
Molybdenum | Stunted growth, leaves appear pale, necrosis in the tissues |
Fig.12. |
Chlorine | Deficiency causes wilting, chlorosis, highly branched root system |
Fig.13. |
Metabolism of Nitrogen
Nitrogen exists as two nitrogen atoms joined triple covalent bond (N ≡ N). The process of conversion of nitrogen into ammonia is known as nitrogen fixation.
There are two methods for the conversion of nitrogen into ammonia. They are as follows-
- Physical nitrogen fixation occurs at the time of lightning. N2 and O2 present in the atmosphere reacts in presence of lightning to form nitric oxide (NO). NO then further gets oxidized to form nitrogen peroxide (NO2).
Formation of ammonia by the decomposition of dead plants and animals is known as ammonification.
The process of conversion of ammonia in nitrite and then into nitrate is known as nitrification. The formation of nitrite occurs in presence of Nitrosomonas bacteria whereas nitrate formation occurs in presence of Nitrobacter.
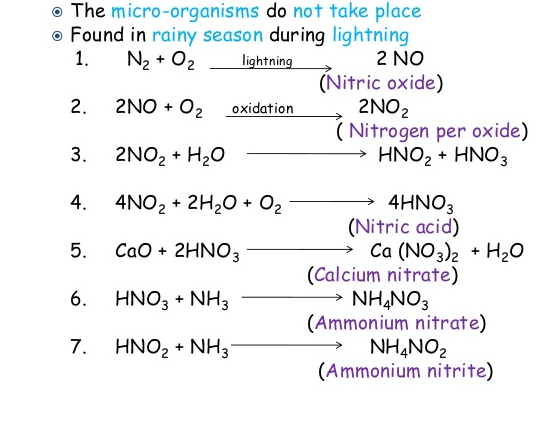
Fig.14. Steps of physical nitrogen fixation
- Biological nitrogen fixation occurs in presence of nitrogen fixing bacteria such as Rhizobium. The prokaryotes that fixes nitrogen biologically are known as diazotrophs. Rhizobium lives in symbiotic association with the roots of the leguminous plants. The enzyme that fixes nitrogen biologically is known as nitrogenase. Nitrogen fixing bacteria can be free living as well as symbiotic. Examples of free-living nitrogen-fixing aerobic microbes are Azotobacter, Rhodospirillum, Anabaena and Nostoc etc.
- lives in symbiotic association with the leguminous plants such as pea, beans, clover, alfalfa, etc. Nitrogen fixation involves the characteristics nodule formation. Nodule formation begins with the interaction of Rhizobium with the roots of the plants. These plants secrete certain chemoattractant that attracts the bacteria towards the roots of the plants. The Rhizobium secrets root hair curling factor that helps the bacteria in invading the roots further. Nitrogenase enzyme that catalyses nitrogen fixation is sensitive towards oxygen. So, leghemoglobin belongs to hemoglobin family serves as oxygen scavenger during nitrogen fixation.
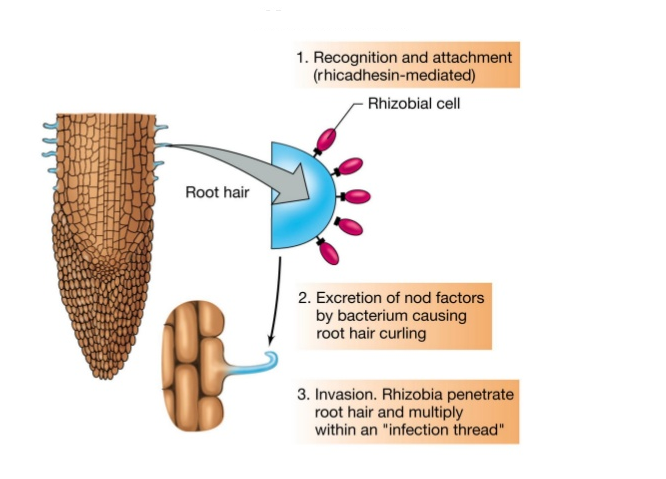
Fig.15. Steps of nodule formation during biological nitrogen fixation
The reaction catalyzed by the nitrogenase is as follows-
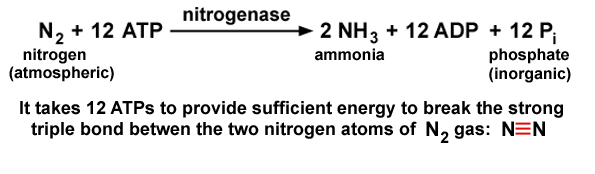
Fig.16. Nitrogenase reaction during biological nitrogen fixation
It is clear from Fig.15, that nitrogen fixation is an energy consuming process. It requires 16 ATP to convert one molecule of nitrogen into two molecules of ammonia.
The ammonia formed after nitrogen fixation is toxic to the plants. Thus, this ammonia has different fate:
- Reductive amination is the process in which ammonia formed reacts with alpha-ketoglutaric acid and forms glutamic acid or glutamate as shown below-
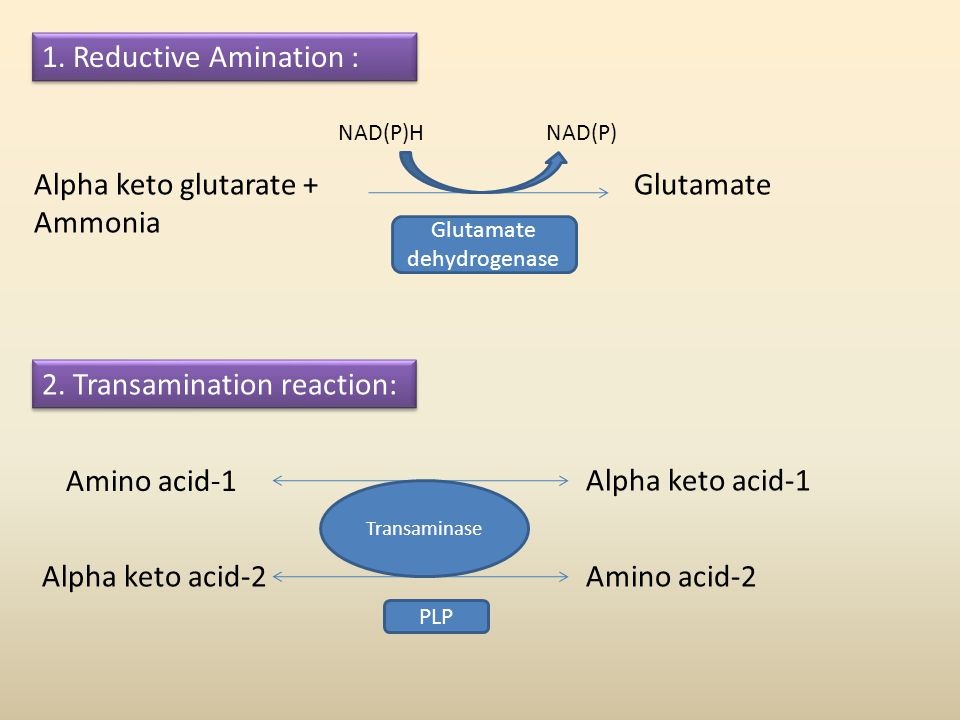
Fig.17. Reductive amination and Transamination
- Transamination involves transfer of amino group from one amino acid to keto acid. This reaction is catalyzed enzymes known as transaminases. This forms asparagine amino acids.
Thus, toxic ammonia is converted into amino acids that are essential for the plant growth and development.












