Respiration in Plants
Table of Content
- Define Respiration
- Glycolysis
- Fermentation/Anaerobic Respiration in Plants
- Krebs Cycle
- Electron Transport Chain (ETC) and Oxidative Phosphorylation
- Amphibolic Pathway
- Respiratory Quotient
- Factors affecting Respiration in Plants
Define Respiration
Respiration or Cellular respiration in plants is a catabolic process that involves the oxidation or breakdown of complex molecules into simple molecules. For example, the breakdown of glucose in presence of oxygen to release carbon-dioxide, water and energy.
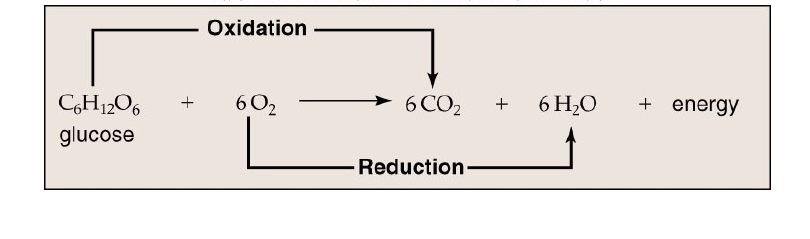
Fig.1. Breakdown of Glucose
The compounds that are oxidized during respiration are known as respiratory substrates, such as, glucose. The energy released during the process is in the form of ATP.
Glycolysis
The process of partial oxidation of glucose in presence of oxygen to form pyruvate is known as glycolysis. It occurs in all living cells in the cytoplasm. The scheme of glycolysis was given by Gustav Embden, Otto Meyerhof, and J. Parnas, So it is often referred to as the EMP pathway.
During glycolysis, one molecule of glucose breakdown to form two molecules of pyruvate. This glucose which undergo glycolysis comes from sucrose, the product of photosynthesis. Sucrose breakdown into glucose and fructose in presence of an enzyme invertase.
Steps of glycolysis are as follows:
- In the first step, glucose gets phosphorylated to form glucose-6-phosphate in presence of the enzyme hexokinase.
- This glucose 6-phosphate the isomerizes to form fructose-6-phosphate in presence of phosphoglucose isomerase.
- Fructose-6-phosphate used is converted into fructose-1,6-biphosphate using phosphofructokinase using ATP as the energy source.
- The fructose 1, 6-bisphosphate converted into dihydroxyacetone phosphate and 3-phosphoglyceraldehyde
- 3-phosphoglyceraldehyde is then converted into 1,3-biphosphoglycerate, to produce NADH.
- 1,3-biphosphoglycerate is converted into 3-phosphoglycerate with the formation of ATP in presence of phosphoglycerate kinase
- 3-phosphoglycerate is then converted into 2-phosphoglycerate in presence of phosphoglycerate mutase.
- 2-phosphoglycerate is converted into phosphoenol pyruvate in presence of enolase.
- Phosphoenol pyruvate is then finally converted into two molecules of pyruvate with the formation of ATP in presence of pyruvate kinase.
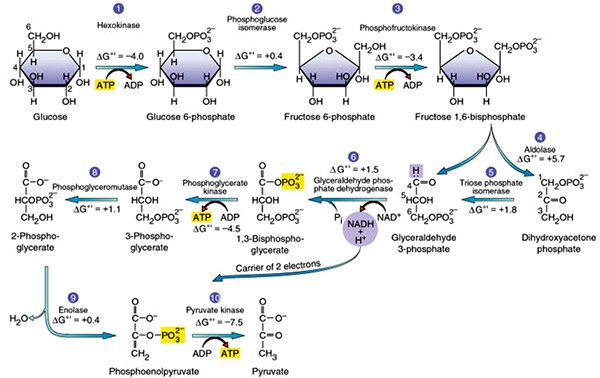
Fig.2. Pathway of Glycolysis
Significance of Glycolysis
It is used as a source of energy for almost all cells.
The product of glycolysis, that is, pyruvate has different fate. In the first case, pyruvate enter into Krebs cycle or citric acid cycle.
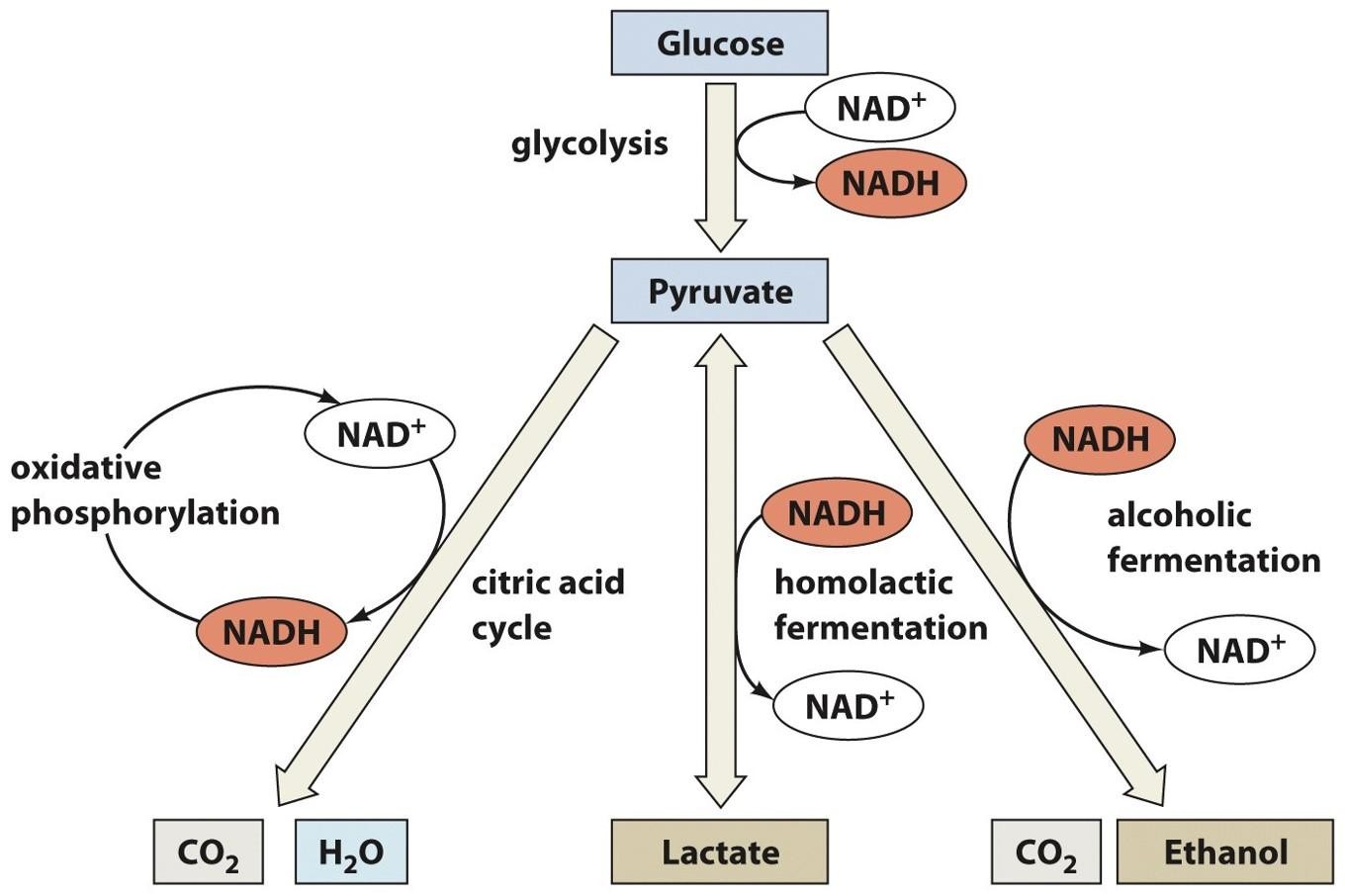
Fig.3. Fate of Pyruvate
In the second case, it can undergo homolactic fermentation to form lactate. Thirdly, it can undergo alcoholic fermentation to form ethanol and carbon-dioxide.
Fermentation/Anaerobic Respiration in Plants
Incomplete oxidation of glucose in absence of oxygen is known as fermentation. The end products are carbon-dioxide and ethanol. During fermentation pyruvate is metabolized into different compounds via different processes as explained below-
- Ethanol fermentation or alcoholic fermentation is the process of formation of ethanol and carbon-dioxide. Ethanol fermentation converts sugars such as glucose, fructose, sucrose into ethanol and carbon-dioxide. This process is used in the formation of alcoholic beverages.

Fig.4. Alcoholic Fermentation
- Lactic acid fermentation refers to the formation of lactic acid. There are two types of lactic acid fermentation-homolactic fermentation and heterolactic fermentation. Homolactic fermentation involves the formation of lactic acid only whereas heterolactic fermentation involves formation of lactic acid as well as other acids and alcohols. Lactic acid fermentation occurs in bacterial cells as well as in muscles during physical work such as exercise.
Krebs Cycle
The product of glycolysis, that is, pyruvate is transported from the cytoplasm into the mitochondria. Pyruvate enters the mitochondrial matrix where oxidative decarboxylation of pyruvate yield acetyl coenzyme A.
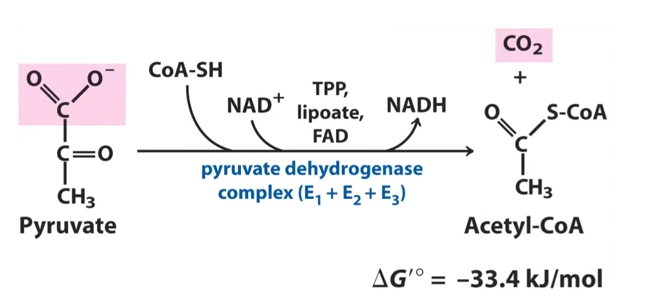
Fig.5.Reaction catalyzed by pyruvate dehydrogenase
Pyruvate dehydrogenase is a complex enzyme made up of three subunits E1, E2 and E3. These three subunits together form a complex. There are different cofactors that are involved during the reaction such as NAD+, FAD, TPP and lipoate.
This acetyl co-enzyme formed enters a cyclic process known as Krebs cycle or tricarboxylic acid cycle or citric acid cycle.
- The citric acid cycle starts with the condensation of acetyl co-enzyme A with oxaloacetic acid to form citric acid in presence of an enzyme citrate synthase.
- Citrate is then isomerized to form isocitrate.
- Isocitrate then undergo two successive decarboxylation reactions to form alpha-ketoglutaric acid and the succinyl coenzyme A with the formation of one molecule of NADH
- Succinyl coenzyme A is converted into succinic acid to form one molecule of GTP. This is substrate level phosphorylation.
- Succinic acid or succinate is then converted into fumarate in presence of an enzyme succinate dehydrogenase
- Fumarate is then converted into malate in presence of an enzyme fumarase with the formation of FADH2
- Malate is the oxidized into oxaloacetic acid in presence of an enzyme malate dehydrogenase with the formation of NADH.
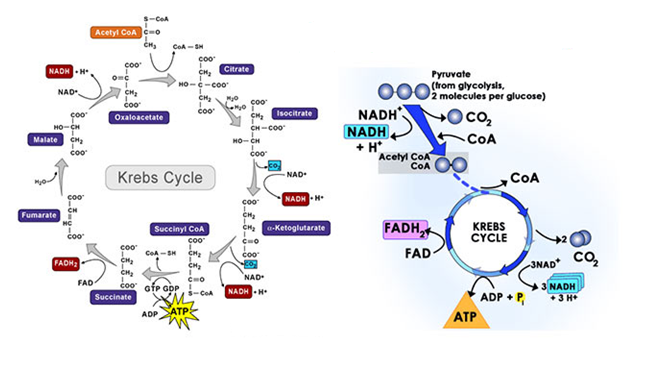
Fig.6. Tricarboxylic acid cycle
The overall reaction of Krebs cycle is given below:
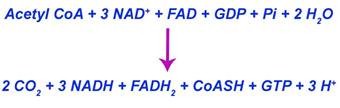
Significance of ATP
It is used to synthesize ATP from the oxidation of glucose, fatty acids and certain amino acids.
Electron Transport Chain (ETC) and Oxidative Phosphorylation
The products of glycolysis, that is, NADH and FADH2 are used to utilize or store energy. NADH and FADH2 are oxidized to release electrons. These electrons are passed to oxygen which results in the formation of water. It involves the different electron carriers that passes electrons which are finally received by oxygen.
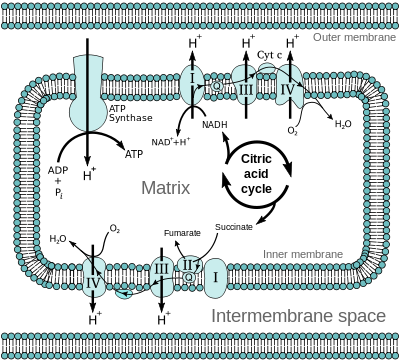
Fig.7. Electron transport chain
Electron carriers are in the form of different complexes. Electrons from NADH produced in the mitochondrial matrix during citric acid cycle are oxidized by a complex I known as NADH dehydrogenase. These electrons are then transferred to ubiquinone located within the inner membrane. Ubiquinone also receives reducing equivalents via FADH2 (complex II) that is generated during oxidation of succinate in the citric acid cycle. The reduced ubiquinone known as ubiquinol which is then oxidized with the transfer of electrons to cytochrome c via complex known as cytochrome bc1 complex (complex III). Cytochrome c is a small protein which acts as a mobile carrier for transfer of electrons between complex III and IV. Complex IV refers to cytochrome c oxidase complex containing cytochromes a and a3, and two copper centers.
The energy released via electron transport is involved in synthesis of ATP. The complex that helps in the formation of ATP is known as ATP synthase. ATP synthase is composed of two major components-F0 and F1.
The F1 is a peripheral membrane protein and is a site where ATP synthesis occurs from ADP and Pi. F0 is an integral membrane protein complex that forms the channel through which protons cross the inner membrane to form a protein gradient. For each ATP produced, 2H+
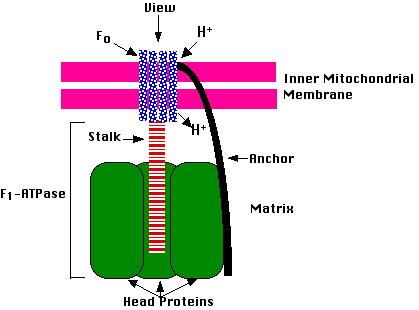
Fig.8. ATP synthase complex
passes through F0 from the intermembrane space to the matrix down the electrochemical proton gradient.
Significance of ETC
The electron transport chain is a system of molecules through which electronsare transferred to generate ATP. It has an important role in both photosynthesis and cellular respiration.
Amphibolic Pathway
Respiratory pathway is known as amphibolic pathway as it involves both anabolism and catabolism.
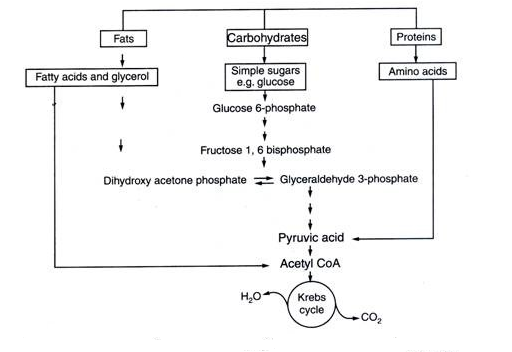
Fig.9. Interrelationship among different metabolic pathways to form carbon-dioxide and water
Respiratory Quotient
The ratio of the volume of CO2 evolved to the volume of O2 consumed in respiration is called the respiratory quotient (RQ) or respiratory ratio. It is given by the formula:
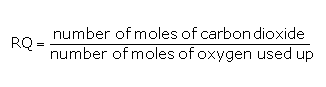
The respiratory quotient depends on the substrate that is used during respiration.
The RQ for different substrate is given below:
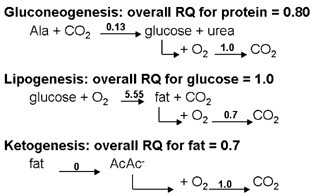
Fig.10. RQ for different substrates for respiration
Factors affecting Respiration in Plants
- Temperature affects the stability of different enzymes so it also affects the respiration. High temperature decreases the rate of respiration. Low temperature does not affect respiration significantly.
- Increase carbon-dioxide decreases the rate of respiration.
- Azide, cyanide are inhibitors of respiration.
- Young plant cells have high rate of respiration.
- More respiratory substrates more is the respiration.