p-BLOCK ELEMENTS – OXYGEN FAMILY
GENERAL CHARACTERISTICS
The elements oxygen, sulphur, selenium, tellurium and polonium belong to group VIA or 16 group of periodic table.
These elements are known as chalcogens i.e. ore forming elements.
ELECTRONIC CONFIGURATION
Elements | At.No. | Electronic confg. | Valence shell electronic confg |
Oxygen | 8 | ||
Sulphur | 16 | ||
Selenium | 34 | ||
Tellurium | 52 | ||
Polonium | 84 |
The oxygen differs from the rest of the elements due to its-
- small size
- higher electronegativity
- absence of d atomic orbitals in valence shell
- tendency to form multiple bonding
METALLIC AND NON METALLIC CHARACTER
Metal (Radio active) t1/2 138.4 days
ABUNDANCE
O > S > Se > Te > Po
Oxygen is the most abundant element. It constitutes 46.6% of earth’s crust, 21% of air and 89.1% of ocean by weight. Sulphur forms about 0.052% of earth’s crust.
DENSITY
Increases down the group regularly.
MELTING POINT AND BOILING POINT
Both show a regular increase down the group due to increase in molecular weight and Van der Waal’s forces of attraction.
OXIDATION STATE
O | S | Se | Te | Po |
–1,–2 | –2 to +6 | –2 to +6 | –2 to +6 | –2 to +6 |
In OF2 the oxidation state of oxygen is +2
IONISATION ENERGY
They possess a large amount of ionisation energy which decrease gradually from O to Po due to increase in size of atoms and increase in screening effect.
ELECTRON AFFINITY
They have high electron affinity which decrease from O to Po. As the size of the atom increases the extra added electron feels lesser attraction by nucleus and electron affinity decreases.
ELECTRONEGATIVITY
It decreases down the group due to decrease in the effective nuclear charge down the group.
CATENATION
The tendency to form chains of identical atoms is known as catenation. It follows the order
S-S > Se – Se > O – O > Te – Te
226 172 142 126 kJ/mol
The higher the bond strength, the higher is the catenation.
ATOMICITY
Oxygen is diatomic, sulphur and selenium octa atomic with puckered ring structure
Ring S6
ALLOTROPY
All the elements exhibit allotropy
Oxygen – O2 dioxygen and O3 ozone
Sulphur – Rhombic (or ) sulphur S8
) sulphur S8
Monoclinic (or ) sulphur S8 (most stable)
) sulphur S8 (most stable)
Plastic (or ) sulphur open chain
) sulphur open chain
Colloidal (or  ) sulphur
) sulphur
The SR changes to SM above 95.4ºC.
Selenium Rhombic Se8 , Monoclinic Se8 (Grey)
Grey is the most stable consisting of regularly arranged spirals of Se atoms.
Tellurium Non metallic, Metallic (more stable)
Polonium  and
and both metallic
both metallic
ATOMIC RADII
Increases regularly from O to Po.
IONIC RADII
Increases regularly from O to Po
ATOMIC VOLUME
Increase regularly from O to Po
MULTIPLE BOND FORMATION
The tendency of these elements to form multiple bonds to C and N decreases down the group eg. S = C = S is moderately stable.
Se = C = Se decomposes readily and
Te = C = Te not known
COMPOUNDS OF SIX GROUP ELEMENTS
HYDRIDES
All these elements form stable hydrides of the type H2M either by directly combining with hydrogen or by the action of acids on metal sulphides, Selenides and tellurides
H2O is a liquid due to hydrogen bonding. Others are colourless gases with unpleasant smell.
H2O > H2S > H2Se > H2Te
104.5º 92.5º 91º 90º (all sp3 hybridised)
The weakening of M – H bond with the increase in the size of M (not the electronegativity) explains the acid character of hydrides.
HALIDES
All these elements form a number of halides. The halides of oxygen are not very stable eg OF2, Cl2O7, I2O5 etc.
HEXAHALIDES
These are formed by fluorine only (not by Cl, Br, I) where elements exhibit maximum valency of +6. SF6, SeF6, TeF6 are colourless gases with sp3d2 hybridisation and octahedral structure. These are covalent in nature. Due to bigger size of Cl, Br and I the coordination number of 6 is not achieved.
TETRAHALIDES
With the exception of SBr4, SI4 and SeI4 all tetrahalides are known. SF4 is gaseous, SeF4 is liquid and TeF4 is solid. SCl4 is unstable liquid. These have lewis acid character.
They have trigonal bipyramidal shape with sp3d hybridization.
DIHALIDES
The dihalides eg SCl2, OF2, TeBr2 are sp3, hybridised and have distorted bond angles due to electron pair repulsions
SCl2 OF2 TeBr2
104º 101.5º 98º
DIMERIC MONOHALIDES
The dimeric monohalides are given by sulphur and selenium eg S2F2, S2Cl2, Se2Cl2 S2Br2, Se2Br2. These are slowly hydrolysed and undergo disproportionation.
The monohalides have structure similar to H2O2 with distorted bond angle of sp3 hybridisation
OXIDES
Ozone is considered as oxide of oxygen O. Oxides of other elements are as follows
Element | Mono Oxide | Dioxide | Tri Oxide |
S | SO | SO2 | SO3 |
Se | – | SeO2 | SeO3 |
Te | TeO | TeO2 | TeO3 |
Po | PoO | PoO2 | – |
SO2 is a gas having sp2 hybridisation and V-shape.
SO3 is a gas, sp2 hybridised and planar in nature.
In solid state it exist as a cyclic trimer (SO3)3 α-form or as a linear chain cross linked sheets
SeO2 volatile solid consists of non planar infinite chains
SeO3 has tetrameric cyclic structure
OXY ACIDS
Sulphur forms four series of oxy acids
SULPHUROUS ACID SERIES
Sulphurous acid (H2SO3)
Thiosulphurous acid (H2S2O2)
Hyposulphurous acid (H2S2O4)
Pyrosulphurous acid (H2S2O5)
SULPHURIC ACID SERIES
Sulphuric acid (H2SO4)

Thiosulphuric acid (H2S2O3)
 or
or 
Pyrosulphuric acid (H2S2O7)

THIONIC ACID SERIES
Dithionic acid (H2S2O6)

Polythionic acid (H2SnO6)

(n=3, 4, 5, 6)
PEROXY ACID SERIES
Peroxomonosulphuric acid (H2SO5)
(Caro’s Acid)
Peroxodisulphuric acid (H2S2O8)
(Marshall’s acid)
Oxyacids of Selenium – Selenous acid (H2SeO3), Selenic acid (H2SeO4)
Oxyacids of Tellurium – Tellurous acid (H2TeO3), Telluric acid (H2TeO4)
OZONE (O3)
Discovered by Van Marum by passing electric discharge through air, named by Schonbein (azo – I smell) and Sorret established the formula O3 as allotrope of oxygen.
It is formed in atmosphere by action of UV rays on O2. It is also formed by-
- slow oxidation of phosphorus in air
- Reaction of fluorine with water at low temperature
- Electrolysis of water
- SO2 reacts with H2O2
PREPARATION
Lab method
By passing silent electric discharge through cold, dry oxygen in ozoniser
By passing silent electric discharge through cold, dry oxygen in ozoniser
The ozonisers used are
- Siemens ozoniser
- Brodie’s ozoniser
MANUFACTURE
For the manufacture of ozonised air Siemen and Halske ozoniser is employed.
Electrolytic method. When acidified water, using high current density and Pt. anode, is electrolysed 95% O3 is obtained at anode rest being O2.
PROPERTIES
- Pale blue gas, dark blue liquid and violet black solid with characteristic strong smell, slightly soluble in water but more soluble in turpentine oil, glacial acetic acid and carbon tetrachloride.
- Decomposition
- Oxidising action
- Reducing action
- Addition reactions
USES
- Bleaching ivory, oils, flour etc.
- As germicide and disinfectant, for sterilising water
- For improving atmosphere in crowded places
- Manufacture of KMnO4 and artificial silk.
TESTS
- Turns starch iodine paper blue.
- Tailing of Hg – Mercury loses its meniscus in contact with O3 and sticks to the surface of glass due to formation of Hg2O
- Clean silver foil blackened by O3
- It turns benzidine paper brown and tetramethyl base violet
STRUCTURE
Oxidation state of O is +1 and –1
OXIDES
Oxides are the binary compounds of oxygen with metals and non metals. Based on their oxygen content they have been classified as-
NORMAL OXIDES
Oxides containing oxygen according to normal oxidation number of M eg. H2O, MgO, Al2O3.
POLYOXIDES
These contain more oxygen than normal oxidation number of M and M – O and O – O bonds. They are further classified as
PEROXIDES
They contain ion, produce hydrogen peroxide with dil. acids and O2 with concentrated acids eg BaO2, Na2O2
SUPER OXIDES
They contain ion. With water they give hydrogen peroxide and oxygen
DIOXIDES
They give chlorine with conc HCl and oxygen with Conc H2SO4 eg. MnO2, PbO2 etc.
SUBOXIDES
They contain lower percentage of oxygen eg. N2O, C3O2
They contain lower percentage of oxygen eg. N2O, C3O2
They have M – M and M – O bonds. eg. O = C = C = C = O (carbon suboxide)
MIXED OXIDES
Formed by the combination of two simple oxides
eg. Red lead, Pb3O4 (PbO2.2PbO), Fe3O4 (FeO+Fe2O3)
Formed by the combination of two simple oxides
eg. Red lead, Pb3O4 (PbO2.2PbO), Fe3O4 (FeO+Fe2O3)
CLASSIFICATION ON THE BASIS OF CHEMICALS BEHAVIOUR
ACIDIC OXIDES
Oxides of non metals which give acids when dissolved in water are called acidic oxides eg.
Oxides of non metals which give acids when dissolved in water are called acidic oxides eg.
CO2, NO2, P2O5, SO2, SO3, Cl2O7 etc.
The metallic oxides of high oxidation state eg Mn2O7, V2O5 and CrO3
BASIC OXIDES
- Ionic in nature. Oxides of alkali and alkaline earth metals eg Na2O, CaO, BaO.
In water they give basic solutions
- Covalent oxides – Oxides of transition metals are covalent in nature eg CuO, FeO. Insoluble in water.
AMPHOTERIC OXIDES
The oxides which react with both acids and alkalis are known as amphoteric oxides eg ZnO, Al2O3, SnO etc.
NEUTRAL OXIDES
Such oxides do not combine with an acid or a base eg NO, N2O, CO, H2O etc.
OXYGEN
Chinese observed the presence of oxygen in air. Priestley & Scheely prepared oxygen by heating suitable oxygen compounds.
OCCURRENCE
About  of the atmosphere is free of elemental oxygen. In the combined state it is present in water 89% by weight, earth’s crust about 50% and plants and animal tissues 50–70%.
of the atmosphere is free of elemental oxygen. In the combined state it is present in water 89% by weight, earth’s crust about 50% and plants and animal tissues 50–70%.
PREPARATION
- By action of heat on oxygen rich compounds
- From oxides
- From peroxides and other oxides
- From certain compounds
- By the action of some chemical reagent on compound rich in O2.
- By electrolysis of water either acidified with H2SO4 using platinum electrodes or by making it alkaline with NaOH or Ba(OH)2 using nickel electrodes.
- By decomposition of steam by chlorine
MANUFACTURE
By fractional distillation of liquid air
PHYSICAL PROPERTIES
It is colourless, odourless, tasteless slightly heavier than air, sparingly soluble in water, soluble in pyrogallol.
CHEMICAL PROPERTIES
On heating it combines directly with metals and non metals
Combination with O2 is accelerated by using catalyst. Platinum is particularly active.
USES
- For breathing
- In welding and cutting – oxy-hydrogen or oxy-acetylene torch is used
- In iron and steel industry – to increase the content of blast in the Bessemer and open hearth process
- As a fuel in rockets
TESTS
- With NO it gives reddish brown fumes of NO2
- Absorbed by alkaline pyrogallol
- A smouldering wood splinter bursts into flames in a jar of O2
STRUCTURE
It is paramagnetic with following electronic configuration
Atomic oxygen : 

Isotopes O16 O17 O18
10000: 1: 8
SULPHUR
OCCURENCE
It occurs free in volcanic regions. In combined state it occurs as
- Gypsum CaSO4.2H2O
- S-Celesite SrSO4
- Galena PbS
- Zinc blende ZnS
- Copper pyrites Cu2SFe2S3
- Iron pyrites FeS2
PROPERTIES
Ordinary sulphur is pale yellow, insoluble in water but dissolves in CS2, C6H6 and turpentine
Allotrophic forms
- Crystalline – Rhombic, monoclinic
- Amorphous – Plastic, milk of sulphur, colloidal sulphur
SULPHURIC ACID (H2SO4)
It is also known as oil of vitriol and king of chemicals.
MANUFACTURE
LEAD CHAMBER PROCESS
The various steps involved are
- Production of SO2 : By burning S or iron pyrites.
- Production of catalyst – Oxides of nitrogen
- Reaction in lead chamber
Gay-Lussac Tower : The residual gases (mainly air + oxides of nitrogen) from lead chambers + conc. H2SO4 from glover tower give nitrated acid.
Glover Tower : The nitrated acid from Gay-Lussac tower is denitrated in this tower.
- Conditions – Temperature 50ºC, excess of steam, lead chamber since lead is not attacked H2SO4.
- Purification – The acid obtained contains the impurities of PbSO4, AS2O3, NO and NO2 which are removed as follows
As2O3 is precipitated as As2S3 by passing H2S
PbSO4 is insoluble in water and settles down on dilution and filtered off.
NO and NO2 are removed by heating with (NH4)2SO4
- Concentration – The sulphuric acid is concentrated by evaporation
CONTACT PROCESS
The steps involved are
- Production of SO2 – It is produced by burning sulphur and iron pyrites and purified by treating with steam to remove dust particles. The arsenic is removed by ferric hydroxide, water vapour removed by conc H2SO4. The gases are filtered through coke filters and purity is tested by Tyndal box.
- Conversion of SO2 to SO3 – It is done in contact or catalyst chamber after being preheated to 450ºC.
450° – 500ºC 1.6 to 1.7 atm Pressure
Catalyst – Formerly platinised asbestos was used which is costly and easily poisoned. Now a days V2O5 is used.
- SO3 is absorbed by conc H2SO4 and then water is added to produce the acid of desired concentration
(oleum or pyrosulphuric acid)
If SO3 is absorbed by water it liberates large amount of heat producing acid fog.
PROPERTIES
- Physical Properties – Its specific gravity is 1.8 and it is 98% by weight normality is 36N.
- Action of heat
Hence it acts as oxidising agent
Non metals are oxidised to their oxides
- Displacement reactions – It displaces volatile acids from their salts.
Metals above hydrogen in ECS react with dil. H2SO4 with evolution of H2.
M(Fe, Sn, Zn, Al, Mn, Mg) + H2SO4 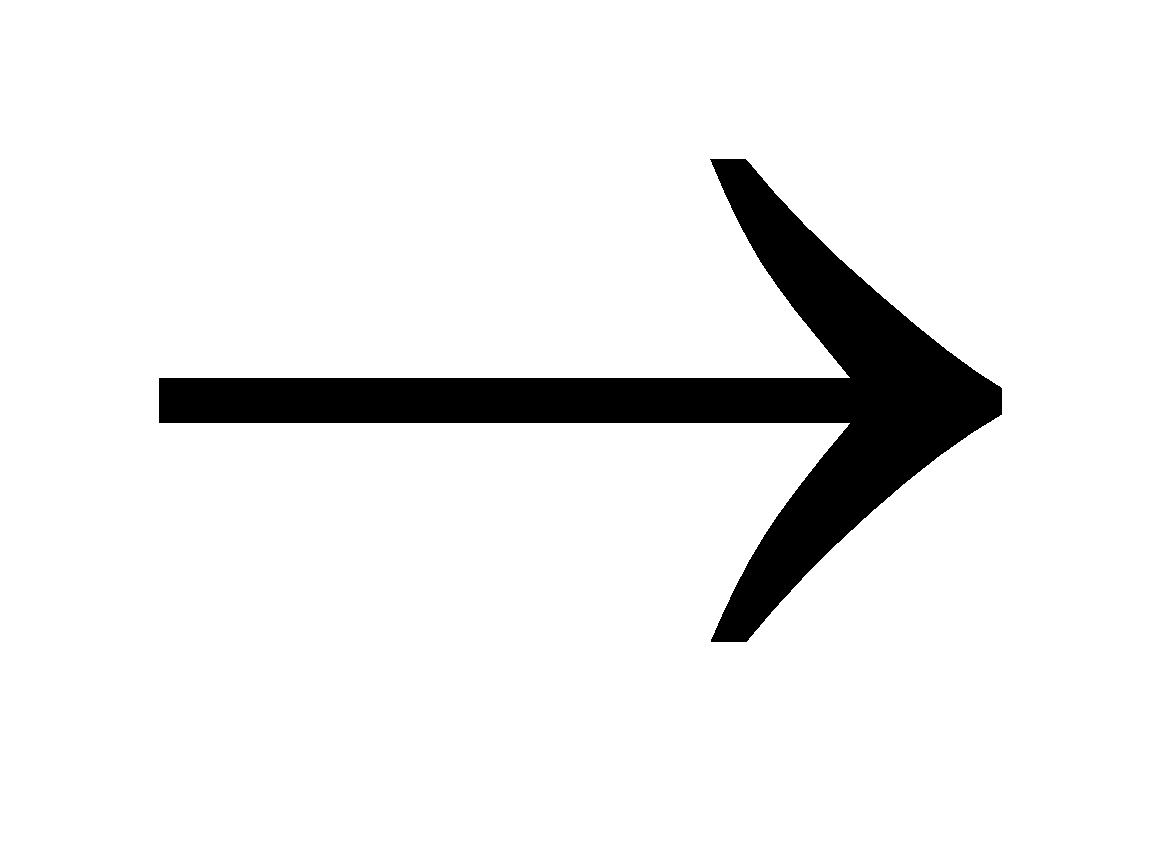 MSO4 + H2
MSO4 + H2
Conc. H2SO4 reacts with all metals except Au and Pt with evolution of SO2.
Metal + Conc. H2SO4  MSO4 + SO2 + H2O
MSO4 + SO2 + H2O
- Dehydrating agent – It is strong dehydrating in nature
- Reaction with PCl5
- Sulfonation of aromatic compounds
Benzene sulphonic acid
- Precipitation – Barium and lead are precipitated as sulphates
Hence these are gravimetrically estimated
Fuming sulphuric acid [H2SO4.SO3]
With ammonia it forms (NH4)2 SO4 hence NH3 can not be dried over H2SO4
USES
It is used as oxidising, dehydrating agent and for the preparation of dyes, drugs, explosives, volatile acids etc.
STRUCTURE
It has tetrahedral structure
s- in doubly excited state
SULPHUR DIOXIDE (SO2)
PREPARATION
- By heating sulphur in air
- Roasting iron pyrites in excess of air
- Lab method : By heating Cu with conc H2SO4
PROPERTIES
- As reducing agent
It decolorises the solution of KMnO4
- As oxidising agent
- Burning of potassium – Potassium burns in SO2 giving sulphate and thiosulphate
- Bleaching action – Its bleaching action is due to reduction
STRUCTURE
It is sp2 hybridised and has V- shape.
S- in excited state
SULPHUR TRIOXIDE (SO3)
PREPARATION
- By heating conc H2SO4 with P2O5
- Manufacture : It is done by contact process
PROPERTIES
It dissolves in water with loud hissing sound and evolution of heat
- Action of heat
- Water
- With
(oleum)
- Acidic nature
- With HCl
(Chloro Sulphonic acid)
- Oxidising agent – It is powerful oxidising agent
STRUCTURE
It has planar trigonal structure due to sp2 hybridisation
S- in excited state
Structure : 
Allotropic forms – It has three allotropic forms 𝛂, 𝞫 and 𝞬
USES
In the manufacture of H2SO4 and drying agent.
HYDROGEN SULPHIDE (H2S)
PREPARATION
By action of dil. H2SO4 or HCl on iron sulphide (Lab method)
PROPERTIES
- Colourless poisonous gas with odour of rotten eggs fairly soluble in water
- Combustibility
- Reducing nature
USES
In qualitative analysis
SODIUM THIOSULPHATE ( )
)
PREPARATION
- By boiling with flowers of sulphur in absence of air
- By heating sodium hydrogen sulphide and sodium hydrogen sulphite together
PROPERTIES
It is also known as hypo
- Complexing – It dissolves silver halides forming complex
Sodium argento thiosulphate (soluble complex)
- It absorbs halogens
Hence used to remove last traces of chlorine
used for estimation of iodine
- Reaction with AgNO3
Silver thiosulphate (white precipitate)
Blue precipitate
- Action of dil. H2SO4
USES
- It is used as an antichlor compound.
- Fixer in photography to remove AgBr left.
- In the metallurgy for extraction of Ag and Au.