Questions
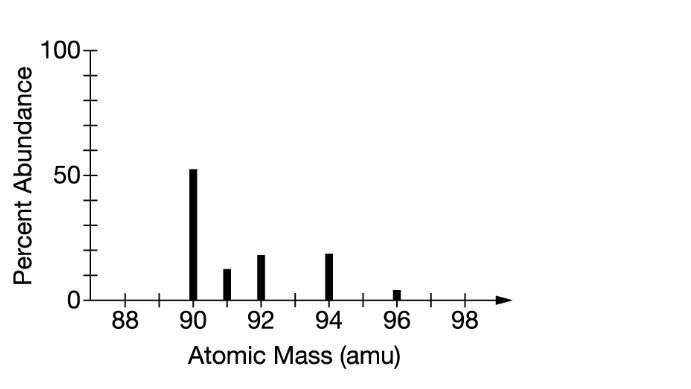
The mass spectrum of an average sample of a pure element is shown in the figure above. Which of the following is the identity of the element?
(A) Y
(B) Zr
(C) Nb
(D) Th
▶️Answer/Explanation
Ans: B
Based on the mass spectrum provided in the image, the element appears to have prominent peaks at atomic mass units of around 90, 92, and 94. This mass distribution pattern is consistent with the natural isotopic abundances of the element Zirconium (Zr).
Zirconium has five naturally occurring isotopes with masses around 90 (Zr-90), 91 (Zr-91), 92 (Zr-92), 94 (Zr-94), and 96 (Zr-96). The most abundant isotopes are Zr-94 and Zr-96, which aligns with the prominent peaks observed in the mass spectrum.
The number of isotopes
The 5 peaks in the mass spectrum shows that there are 5 isotopes of zirconium – with relative isotopic masses of \(90,91,92,94\) and 96 on the \({ }^{12} \mathrm{C}\) scale.
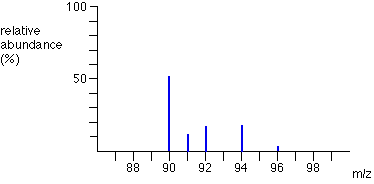
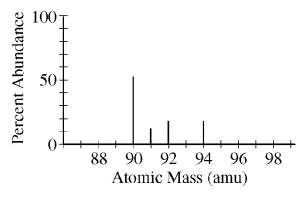
Question
The mass spectrum for an unknown element is shown above. According to the information in the spectrum, the atomic mass of the unknown element is closest to
A 90amu
B 91amu
C 93amu
D 94amu
▶️Answer/Explanation
Ans:B
The relative abundances of the isotopes with atomic masses 90 and 92 have a weighted average that would be less than 91. That result with the influence of the mass and abundance of the isotope with atomic mass 94 would result in a weighted average closer to 91 than to 93.
Question
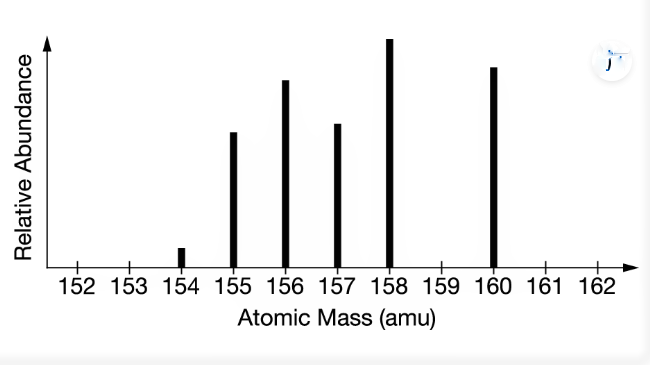
The mass spectrum represented above is most consistent with which of the following elements?
A Eu
B Gd
C Tb
D Dy
▶️Answer/Explanation
Ans:B
The average atomic mass of Gd is close to 157amu. The relative abundance and masses of the isotopes shown in the mass spectrum predict an average atomic mass close to 157amu.