Multiple Choice Questions
MCQs
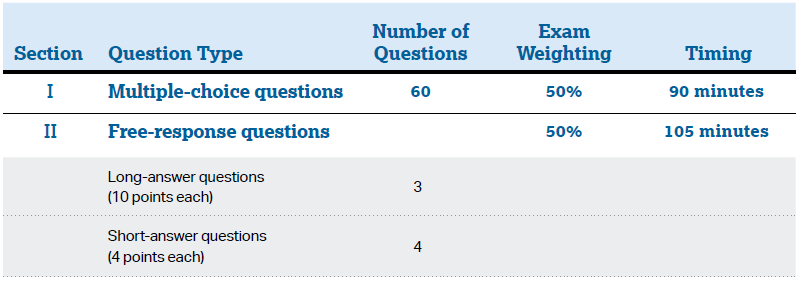
- Time: 90 minutes
- 60 multiple – choice questions (core)
- No marks deducted from incorrect answers
- A four-function, scientific, or graphing calculator is allowed
- 50% weight
Free-Response Questions
- Time: 105 minutes
- 4 Questions
- No marks deducted from incorrect answers
- A four-function, scientific, or graphing calculator is allowed
- 50% weight
Unit 1: Atomic Structure and Properties
Unit 2: Molecular and Ionic Compound Structure and Properties
Unit 3: Intermolecular Forces and Properties
- 3.1 Intermolecular Forces
- 3.2 Properties of Solids
- 3.3 Solids, Liquids, and Gases
- 3.4 Ideal Gas Law
- 3.5 Kinetic Molecular Theory
- 3.6 Deviation from Ideal Gas Law
- 3.7 Solutions and Mixtures
- 3.8 Representations of Solutions
- 3.9 Separation of Solutions and Mixtures Chromatography
- 3.10 Solubility
- 3.11 Spectroscopy and the Electromagnetic Spectrum
- 3.12 Photoelectric Effect
- 3.13 Beer-Lambert Law
Unit 4: Chemical Reactions
Unit 5: Kinematics
- 5.1 Reaction Rate
- 5.2 Introduction to Rate Law
- 5.3 Concentration Change Over Time
- 5.4 Elementary Reactions
- 5.5 Collision Model
- 5.6 Reaction Energy Profile
- 5.7 Introduction to Reaction Mechanisms
- 5.8 Reaction Mechanism and Rate Law
- 5.9 Steady State Approximation
- 5.10 Multisteps Reaction Energy profile
- 5.11 Catalyst
Unit 7: Equilibrium
- 7.1 Introduction to Equilibrium
- 7.2 Direction of Reversible Reactions
- 7.3 Reaction Quotient and Equilibrium Constant
- 7.4 Calculating the Equilibrium Constant
- 7.5 Magnitude of the Equilibrium Constant
- 7.6 Properties of the Equilibrium Constant
- 7.7 Calculating Equilibrium Concentrations
- 7.8 Representations of Equilibrium
- 7.9 Introduction to Le Châtelier’s Principle
- 7.10 Reaction Quotient and Le Châtelier’s Principle
- 7.11 Introduction to Solubility Equilibria
- 7.12 Common-Ion Effect
- 7.13 pH and Solubility
- 7.14 Free Energy of Dissolution
Unit 8: Acids and Bases
- 8.1 Introduction to Acids and Bases
- 8.2 pH and pOH of Strong Acids and Bases
- 8.3 Weak Acid and Base Equilibria
- 8.4 Acid-Base Reactions and Buffers
- 8.5 Acid-Base Titrations
- 8.6 Molecular Structure of Acids and Bases
- 8.7 pH and pKa
- 8.8 Properties of Buffers
- 8.9 Henderson-Hasselbalch Equation
- 8.10 Buffer Capacity
Unit 9: Applications of Thermodynamics
- 9.1 Introduction to Entropy
- 9.2 Absolute Entropy and Entropy Change
- 9.3 Gibbs Free Energy and Thermodynamic Favorability
- 9.4 Thermodynamic and Kinetic Control
- 9.5 Free Energy and Equilibrium
- 9.6 Coupled Reactions
- 9.7 Galvanic (Voltaic) and Electrolytic Cells
- 9.8 Cell Potential and Free Energy
- 9.9 Cell Potential Under Nonstandard Conditions
- 9.10 Electrolysis and Faraday’s Law
Free-Response Questions(FRQs)
MCQs
- Time: 90 minutes
- 60 multiple – choice questions (core)
- No marks deducted from incorrect answers
- A four-function, scientific, or graphing calculator is allowed
- 50% weight
Free-Response Questions
- Time: 105 minutes
- 4 Questions
- No marks deducted from incorrect answers
- A four-function, scientific, or graphing calculator is allowed
- 50% weight
Unit 1: Atomic Structure and Properties
Unit 2: Molecular and Ionic Compound Structure and Properties
Unit 3: Intermolecular Forces and Properties
- 3.1 Intermolecular Forces
- 3.2 Properties of Solids
- 3.3 Solids, Liquids, and Gases
- 3.4 Ideal Gas Law
- 3.5 Kinetic Molecular Theory
- 3.6 Deviation from Ideal Gas Law
- 3.7 Solutions and Mixtures
- 3.8 Representations of Solutions
- 3.9 Separation of Solutions and Mixtures Chromatography
- 3.10 Solubility
- 3.11 Spectroscopy and the Electromagnetic Spectrum
- 3.12 Photoelectric Effect
- 3.13 Beer-Lambert Law
Unit 4: Chemical Reactions
Unit 5: Kinematics
- 5.1 Reaction Rate
- 5.2 Introduction to Rate Law
- 5.3 Concentration Change Over Time
- 5.4 Elementary Reactions
- 5.5 Collision Model
- 5.6 Reaction Energy Profile
- 5.7 Introduction to Reaction Mechanisms
- 5.8 Reaction Mechanism and Rate Law
- 5.9 Steady State Approximation
- 5.10 Multisteps Reaction Energy profile
- 5.11 Catalyst
Unit 7: Equilibrium
- 7.1 Introduction to Equilibrium
- 7.2 Direction of Reversible Reactions
- 7.3 Reaction Quotient and Equilibrium Constant
- 7.4 Calculating the Equilibrium Constant
- 7.5 Magnitude of the Equilibrium Constant
- 7.6 Properties of the Equilibrium Constant
- 7.7 Calculating Equilibrium Concentrations
- 7.8 Representations of Equilibrium
- 7.9 Introduction to Le Châtelier’s Principle
- 7.10 Reaction Quotient and Le Châtelier’s Principle
- 7.11 Introduction to Solubility Equilibria
- 7.12 Common-Ion Effect
- 7.13 pH and Solubility
- 7.14 Free Energy of Dissolution
Unit 8: Acids and Bases
- 8.1 Introduction to Acids and Bases
- 8.2 pH and pOH of Strong Acids and Bases
- 8.3 Weak Acid and Base Equilibria
- 8.4 Acid-Base Reactions and Buffers
- 8.5 Acid-Base Titrations
- 8.6 Molecular Structure of Acids and Bases
- 8.7 pH and pKa
- 8.8 Properties of Buffers
- 8.9 Henderson-Hasselbalch Equation
- 8.10 Buffer Capacity
Unit 9: Applications of Thermodynamics
- 9.1 Introduction to Entropy
- 9.2 Absolute Entropy and Entropy Change
- 9.3 Gibbs Free Energy and Thermodynamic Favorability
- 9.4 Thermodynamic and Kinetic Control
- 9.5 Free Energy and Equilibrium
- 9.6 Coupled Reactions
- 9.7 Galvanic (Voltaic) and Electrolytic Cells
- 9.8 Cell Potential and Free Energy
- 9.9 Cell Potential Under Nonstandard Conditions
- 9.10 Electrolysis and Faraday’s Law
Course Content
The AP Chemistry Exam assesses student understanding of the science practices and learning objectives outlined in the course framework. The exam is 3 hours and 15 minutes long and includes 60 multiple-choice questions and 7 free-response questions. Starting with the 2022–23 school year (spring 2023 exam), a scientific or graphing calculator is recommended for use on both sections of the exam. Students are provided with the periodic table and a formula sheet that lists specific and relevant formulas for use on the exam
Units Exam Weighting
Unit 1: Atomic Structure and Properties 7–9%
Unit 2: Molecular and Ionic Compound Structure and Properties 7–9%
Unit 3: Intermolecular Forces and Properties 18–22%
Unit 4: Chemical Reactions 7–9%
Unit 5: Kinetics 7–9%
Unit 6: Thermodynamics 7–9%
Unit 7: Equilibrium 7–9%
Unit 8: Acids and Bases 11–15%
Unit 9: Applications of Thermodynamics 7–9%