Question
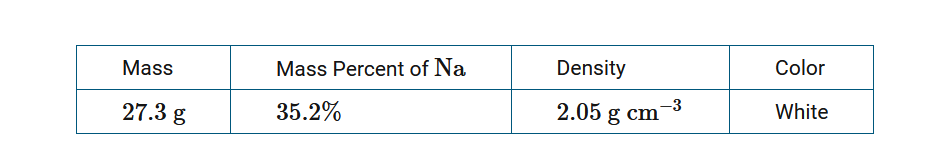
A jar labeled NaCl contains a powder. The table above contains information determined by analyzing a sample of the powder in the laboratory. What information in the table is the most helpful in determining whether the powder is pure NaCl?
D Color
▶️Answer/Explanation
Ans:B
Percent by mass is an intensive property that is determined by the composition of a substance and therefore is helpful for determining the purity of the powder.
Question
A student obtains a mixture of the chlorides of two unknown metals, X and Z. The percent by mass of X and the percent by mass of Z in the mixture is known. Which of the following additional information is most helpful in calculating the mole percent of XCl(s) and of ZCl(s) in the mixture?
A The number of isotopes of Cl
B The molar masses of X and Z
C The density of either XCl(s) or ZCl(s)
D The percent by mass of Cl in the mixture
▶️Answer/Explanation
Ans: B
The molar mass \(\frac{mass}{moles}\) is the quantity that establishes the ratio of the mass of a pure substance in a mixture to its number of moles. This ratio can be used to calculate the mole percent of either XCl or ZCl in the mixture.
Question
A vessel contains a mixture of gases. The mass of each gas used to make the mixture is known. Which of the following information is needed to determine the mole fraction of each gas in the mixture?
D The number of atoms per molecule for each gas
▶️Answer/Explanation
Ans: A
The mole fraction of each gas is equal to the number of moles of the gas divided by the total number of moles of gases in the mixture. Given the mass of each gas, the molar mass of each gas is needed to determine the number of moles for each gas and the total number of moles in the mixture.
Refer to the following information.
\(Cu(s)+4HNO_{3}(aq)\rightarrow Cu(NO_{3})(aq)+NO_{2}(g)+2H_{2}O(l)\)
Each student in a class placed a 2.00 g sample of a mixture of Cu and Al in a beaker and placed the beaker in a fume hood. The students slowly poured 15.0 mL of 15.8 M HNO3(aq) into their beakers. The reaction between the copper in the mixture and the HNO3(aq) is represented by the equation above. The students observed that a brown gas was released from the beakers and that the solutions turned blue, indicating the formation of \(Cu^{2+}\)(aq). The solutions were then diluted with distilled water to known volumes.
Question.
. The students determined that the reaction produced 0.010 mol of\( Cu(NO_{3})_{2}\). Based on the measurement, what was the percent of Cu by mass in the original 2.00 g sample of the mixture?
(B) 32%
(C) 64%
(D) 96%
▶️Answer/Explanation
Ans:A
Question
A combination of sand, salt, and water is an example of a __________.
A) heterogeneous mixture
B) solid
C) pure substance
D) compound
E) homogeneous mixture
▶️Answer/Explanation
Ans: A
