Question
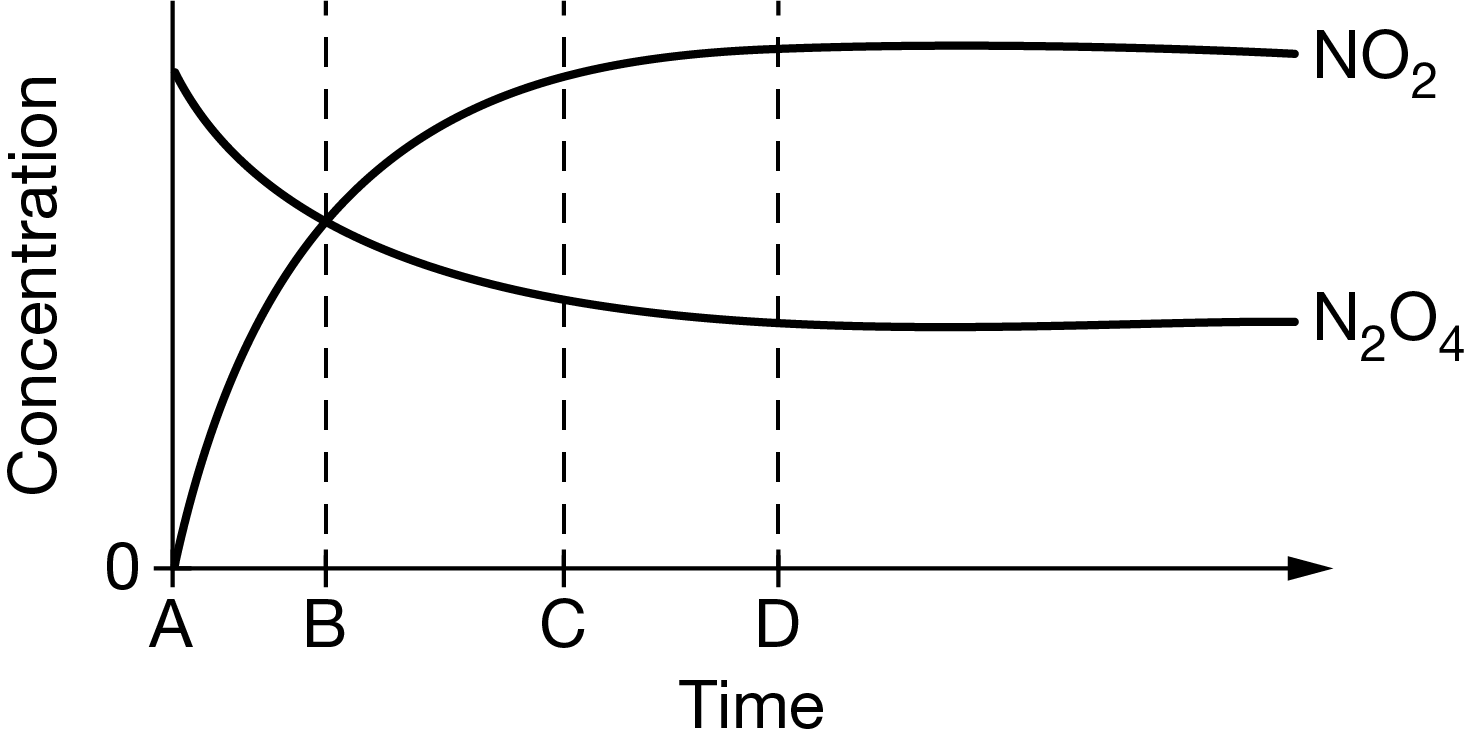
\(N_2O_4(g)\)⇄\(2NO_2(g)\)
The graph above represents the data collected under certain conditions for the decomposition of \(N_2O_4(g)\) according to the chemical equation above. Based on the graph, at approximately which time is equilibrium established?
A At time A , because \(N_2O_4(g)\) is expanding to fill the container.
B At time B , because the reaction is reversible and \([NO_2]=[N_2O_4]\) .
C At time C , because the reaction is about to reach completion and \([NO_2]>[N_2O_4]\) .
D At time D , because there are no observable changes in \([NO_2]\) and \([N_2O_4]\).
▶️Answer/Explanation
Ans:D
At equilibrium, \(N_2O_4(g)\) is transformed into NO2 at the same rate at which NO2 is transformed into \(N_2O_4(g)\), and no changes in \([NO_2]\) and \([N_2O_4]\) are observed.
Question
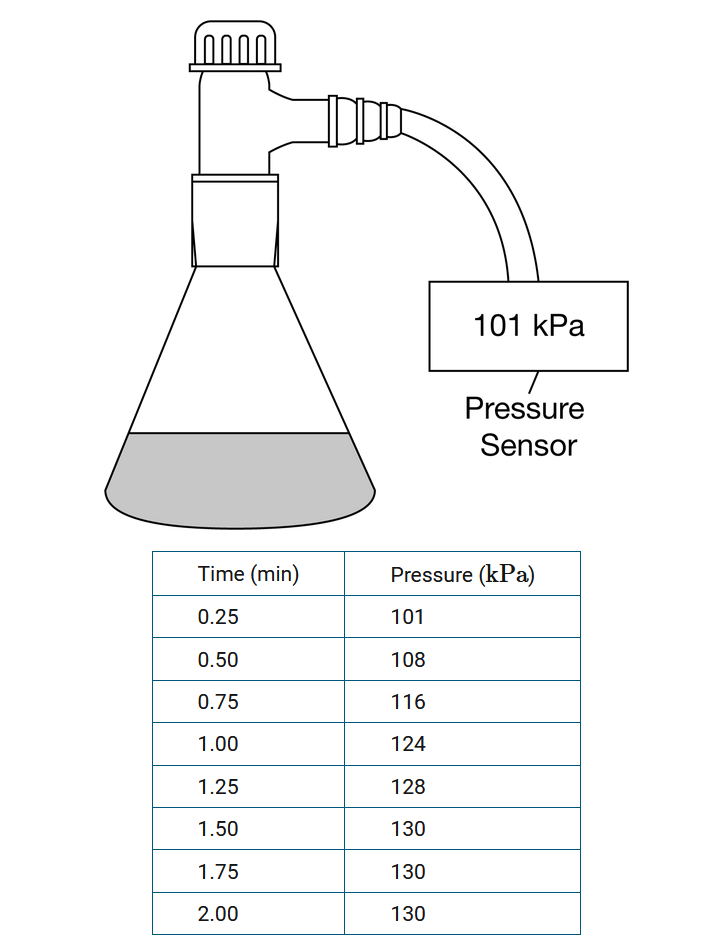
A sample of acetone is placed into a container. The container is sealed and attached to a pressure sensor, as shown in the diagram above. The container is allowed to sit on the lab table for a few minutes as the pressure in the container is monitored at regular intervals. At the end of 2.00 minutes, some acetone liquid remains in the container. Which of the following best explains the pressure data presented in the table above?
A The acetone heats up over time, causing more of it to vaporize at an increasing rate.
B The acetone has completely vaporized after 1.50 minutes, so the pressure becomes constant.
C The acetone vaporizes from the liquid at a constant rate, the rate of condensation increases until it becomes equal to the rate of evaporation, and then the pressure stays constant.
D The acetone vaporizes from the liquid at a rate that is fast in the beginning but then slows down until the vaporization process stops completely.
▶️Answer/Explanation
Ans:C
A constant equilibrium pressure is achieved once the rate of condensation of the acetone vapor matches the rate of evaporation of the acetone from the liquid.
Question
Brown Colorlesss
\(2NO_2(g)\)⇄\(N_2O_4(g)\)
A sample of pure \(NO_2(g)\) in a sealed tube at 20°C is placed in a temperature bath at 30°C. Observations of changes in the color, pressure, and mass of the mixture are recorded as a function of time. Which of the following is an observation that would best support the claim that the reaction represented above has reached equilibrium at 30°C ?
A The total mass of the system remains constant because the amounts of reactant and product do not change with time at equilibrium.
B The color of the system changes from brown to completely colorless because only the product will be present at equilibrium.
C The total pressure of the system decreases then reaches a constant value because the amounts of reactant and product no longer change at equilibrium.
D The temperature of the system remains constant because the temperature must be constant at equilibrium.
▶️Answer/Explanation
Ans:C
As the reaction proceeds to the right, two \(NO_2\) molecules are converted into one \(N_2O_4(g)\) molecule, resulting in a decrease in the number of molecules in a fixed volume and a decrease in pressure. At equilibrium, the pressure will be constant since the number of reactant and product molecules will be constant.
Question
At equilibrium, __________.
A) the rates of the forward and reverse reactions are equal
B) the rate constants of the forward and reverse reactions are equal
C) all chemical reactions have ceased
D) the value of the equilibrium constant is 1
E) the limiting reagent has been consumed
▶️Answer/Explanation
Ans: A
Question
Which one of the following will change the value of an equilibrium constant?
A) adding other substances that do not react with any of the species involved in the equilibrium
B) varying the initial concentrations of reactants
C) changing temperature
D) varying the initial concentrations of products
E) changing the volume of the reaction vessel
▶️Answer/Explanation
Ans: C
