Question
\(PC1_3(g) + Cl_2(g) \rightleftharpoons PCl_{5}(g)\) \( K_{c} =6.5\)
At a certain point in time, a 1.00 L rigid reaction vessel contains 1.5 mol of PCl,(g), 1.0 mol of \(Cl_2\)(g), and 2.5 mol of\( PCl_5\)(g). Which of the following describes how the measured pressure in the reaction vessel will change and why it will change that way as the reaction system approaches equilibrium at constant temperature?
(A) The pressure will increase because \(Q< K_c\)
(B) The pressure will increase because \(Q > K_c\)
(C) The pressure will decrease because \(Q< K_c\)
(D) The pressure will decrease because \(Q > K_c\)
▶️Answer/Explanation
Ans:C
Question
\(3O_{2}(g)\rightleftharpoons 2O_{3}(g) \) \(K_{c}=1.8\times 10^{-56}\)at 570k
For the system represented above, [\(O_{2}\)] and \([O_{3}]\) initially are 0.150 mol/L and 2.5 mol/L respectively. Which of the following best predicts what will occur as the system approaches equilibrium at 570 K?
(A) The amount of\( O_{3}\)(g) will increase, because Q<K
(B) The amount of \(O_{3}\)(g) will decrease, because Q<K
(C) The amount of \(O_{3}\)(g) will increase, because Q> K
(D) The amount of \(O_{3}\)(g) will decrease, because Q>K
▶️Answer/Explanation
Ans:C
Question
The value of \(K_{eq}\) for the following reaction is 0.25:
\(SO_2(g) + NO_2(g) \leftrightarrow SO_3(g) + NO(g)\)
The value \(K_{eq}\) at the same temperature for the reaction below is _____.
\(2SO_2(g) + 2NO_2(g) \leftrightarrow 2SO_3(g) + 2SO_3(g) + 2NO (g)\)
A) 0.062
B) 16
C) 0.25
D) 0.50
E) 0.12
▶️Answer/Explanation
Ans: A
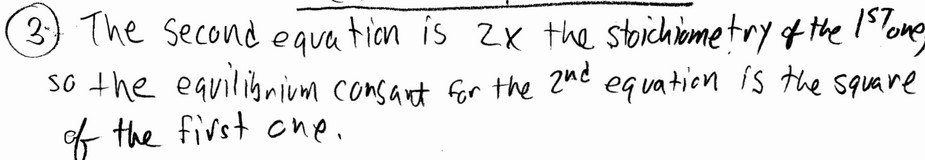
Question
The value of \(K_{eq}\) for the equilibrium
\(H_2(g) + I_2(g) \leftrightarrow 2HI(g)\)
is 794 at \(25^o\) C. At this temperature, what is the value of \(K_{eq}\) for the equilibrium below?
\(HI(g) \leftrightarrow 1/2 H_2(g) + 1/2I_2(g)\)
A) 0.035
B) 0.0013
C) 28
D) 397
E) 1588
▶️Answer/Explanation
Ans: A

