Question
\(CaF_{2}(s)\rightleftharpoons Ca^{2+}(aq)+2F^{-}(aq)\) \(\Delta H>0\)
Dissolution of the slightly soluble salt \(CaF_{2}\) is shown by the equation above. Which of the following changes will decrease \([Ca^{2+}] \)in a saturated solution of \(CaF_{2}\) , and why? (Assume that after each change some \(CaF_{2}\)(s) remains in contact with the solution.)
(A) Allowing some of the water to evaporate from the solution, because more \(CaF_{2}\)(s)
will precipitate
(B) Adding 0.1 M HNO3(aq), because some \(F^{−}\)(aq) ions will become protonated
(C) Adding 0.1 M \(NaNO_{3}\)(aq), because additional liquid will dilute the solution
(D) Adding NaF(s), because the reaction will proceed toward reactants
▶️Answer/Explanation
Ans:D
The given equilibrium dissolution reaction is:
\[ CaF_{2}(s) \rightleftharpoons Ca^{2+}(aq) + 2F^{-}(aq) \quad \Delta H > 0 \]
To decrease the concentration of \( Ca^{2+} \) ions (\( [Ca^{2+}] \)) in a saturated solution of \( CaF_{2} \), we need to shift the equilibrium position of the dissolution reaction to the left, favoring the formation of solid \( CaF_{2}(s) \). Let’s analyze each option:
(A) Allowing some of the water to evaporate from the solution, because more \( CaF_{2}(s) \) will precipitate:
This option would actually increase the concentration of \( Ca^{2+} \) ions because it would decrease the volume of the solution, leading to an increase in the concentration of all dissolved species, including \( Ca^{2+} \) ions. Therefore, option (A) is incorrect.
(B) Adding 0.1 M HNO3(aq), because some \( F^{-}(aq) \) ions will become protonated:
The addition of nitric acid (\( HNO_{3} \)) would protonate some of the fluoride ions (\( F^{-} \)), leading to the formation of HF. However, this would not directly affect the concentration of \( Ca^{2+} \) ions or shift the equilibrium position of the dissolution reaction. Therefore, option (B) is incorrect.
(C) Adding 0.1 M \( NaNO_{3} \)(aq), because additional liquid will dilute the solution:
Adding sodium nitrate (\( NaNO_{3} \)) would not directly affect the concentration of \( Ca^{2+} \) ions or shift the equilibrium position of the dissolution reaction. While the solution would be diluted, this would not change the solubility equilibrium. Therefore, option (C) is incorrect.
(D) Adding NaF(s), because the reaction will proceed toward reactants:
Adding solid sodium fluoride (\( NaF(s) \)) would introduce additional fluoride ions (\( F^{-} \)) into the solution. According to Le Chatelier’s principle, increasing the concentration of fluoride ions would shift the equilibrium position of the dissolution reaction to the left, favoring the formation of solid \( CaF_{2}(s) \) and decreasing the concentration of \( Ca^{2+} \) ions. Therefore, option (D) is the correct answer.
Thus, the correct answer is:
(D) Adding NaF(s), because the reaction will proceed toward reactants.
Question
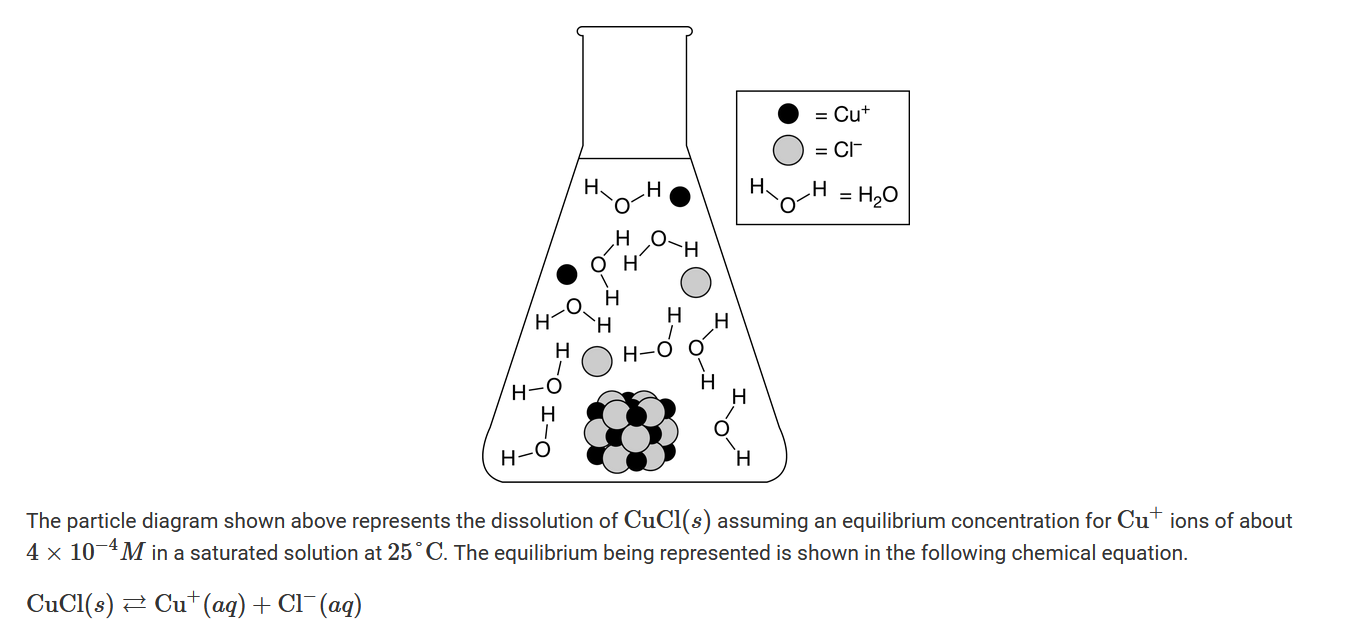
Which of the following best explains what the particle diagram is able to show about the entropy change for the dissolution of \(CuCl(s)\) in pure water?
A The particle diagram shows that the dissociation of \(CuCl(s)\) into ions contributes to an increase in the entropy for the dissolution.
B The particle diagram shows that the dissociation of \(CuCl(s)\) into ions contributes to a decrease in the entropy for the dissolution.
C The particle diagram shows that there is no reorganization of the water molecules around the ions and the change in entropy for the dissolution is zero.
D The particle diagram shows that there are no interactions between the water molecules and the change in entropy for the dissolution is zero.
▶️Answer/Explanation
Ans:A
As a result of the dissociation of \(CuCl(s)\) , two particles form, \(Cu^+\) and \(Cl^−\). This is shown in the particle diagram, and this increase in the number of particles contributes to an increase in entropy.
Question

Which of the following best explains whether or not the particle diagram can predict the relative value of the enthalpy change for the dissolution of \(CuCl\)(s)?
A The value of the enthalpy change for the dissolution of \(CuCl\)(s) cannot be predicted from the particle diagram because it fails to illustrate the amount of energy required to overcome the forces between solute particles and between solvent particles.
B The value of the enthalpy change for the dissolution of \(CuCl\)(s) cannot be predicted from the particle diagram because it fails to illustrate the amount of energy released when the water molecules form hydrogen bonds with Cl− ions.
C The value of the enthalpy change for the dissolution of \(CuCl\)(s) is positive (endothermic) because energy is released to overcome the forces between solute particles, as shown in the particle diagram.
D The value of the enthalpy change for the dissolution of \(CuCl\)(s) is negative (exothermic) because energy is required when the bonds between the ions are broken, as shown in the particle diagram.
▶️Answer/Explanation
Ans:A
The particle diagram does not provide enough information to predict if the dissolution of \(CuCl\) is endothermic or exothermic. The enthalpy change for the dissolution can be evaluated only if the relative energy of these processes are known: (1) the energy required to overcome the interactions between solute particles, (2) the energy required to overcome the interactions between solvent particles, and (3) the energy released when new solute-solvent interactions are formed.
Question
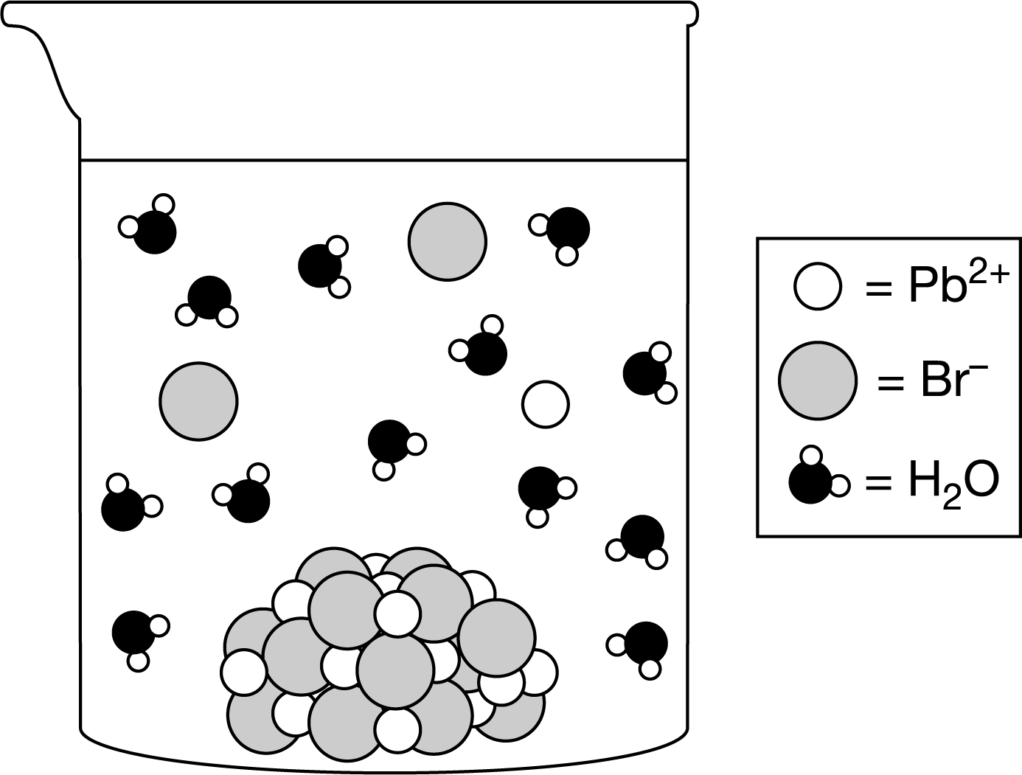
Shown above are a chemical equation that represents the dissolution of \(PbBr_2\) in pure water, a table of the changes in some thermodynamic properties for the process, and a particle diagram. Which of the following explains which relative change in a thermodynamic property is best illustrated by the particle diagram?
A The reorganization of the water molecules around the ions illustrates that ΔH°>0 because forming strong ion-dipole interactions releases energy.
B The very small amount of \(Pb^{2+}\) and \(Br^−\) ions illustrates that ΔS°>0 because entropy decreases when \(PbBr_2\)dissolves.
C The very small amount of \(Pb^{2+}\) and \(Br^−\) ions illustrates that ΔG°>0 because the dissolution of \(PbBr_2\)is not a favorable process.
D The very large amount of solid that remains undissolved illustrates that ΔG°>0 because the dissolution of \(PbBr_2\)is a favorable process.
▶️Answer/Explanation
Ans:C
The relatively small amount of \(Pb^{2+}\) and \(Br^−\) ions shown in the particle diagram best illustrates that positive ΔG°>0, which indicates that the dissolution of PbBr2 is not a favorable process (not spontaneous) under standard conditions, so its solubility is low \((K_{sp}<<1)\).
Question
A student observes that the equilibrium constant for a reaction is greater than 1.0 at temperatures below 500 K but less than 1.0 at temperatures above 500 K. What can the student conclude
about the values of \(\Delta H^{\circ}\) and \(\Delta S^{\circ}\) for the reaction? (Assume that \(\Delta H^{\circ}\) and \(\Delta S^{\circ} \)are independent of temperature.)
(A) \(\Delta H^{\circ} > 0 ~and ~\Delta S^{\circ}\)
(B) \(\Delta H^{\circ} > 0 ~and ~\Delta S^{\circ}\)
(C) \(\Delta H^{\circ} > 0~ and~ \Delta S^{\circ}\)
(D) \(\Delta H^{\circ} > 0 ~and ~ \Delta S^{\circ}\)
▶️Answer/Explanation
Ans:D
Question
A group of students was asked to recover Cu(s) from a blue-green aqueous solution containing an unknown concentration of \(Cu^{2+}\)(aq). The students took a 100.0 mL sample of the solution and added an excess of 1.0 M \(Na_3PO_4\)(aq), causing the\( Cu^{2+}\)(aq) to precipitate as \(Cu_3(PO_4)_2(s)\), as shown in step 1 below.
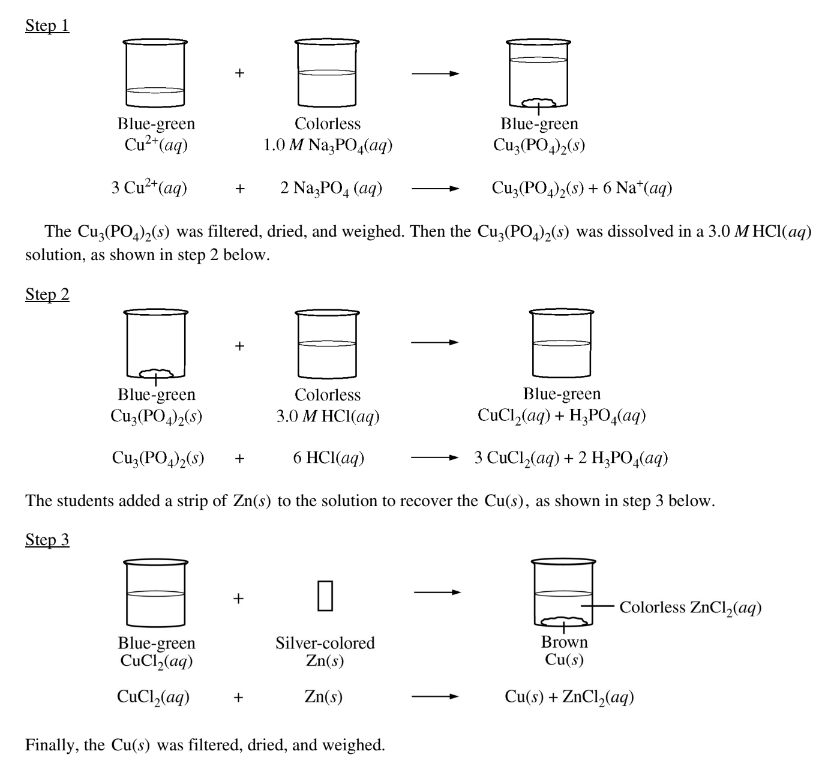
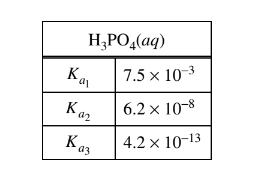
The values of the dissociation constants for \(H_{3}PO_{4}\) are given in the table above. Given thatmthe solution in the beaker at the end of step 2 had a pH of approximately 1, which of the following chemical species had the lowest concentration among the products of step 2 ?
(A) \( H^{+}\)(aq)
(B) \(H_{2}PO_{4 }^{-}(aq) \)
(C) \(HPO_{4}^{2−}\)(aq)
(D) \(PO_{4}^{3−}\)(aq)
▶️Answer/Explanation
Ans:C