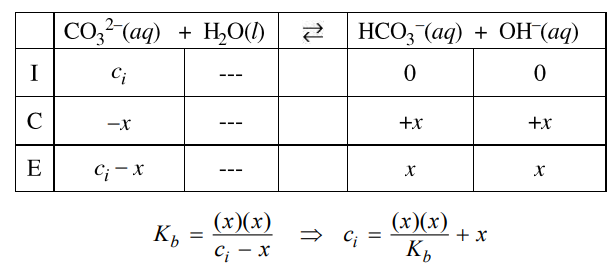Question
N2(g) + O2(g) ⇔2 NO(g)
At high temperatures, N2(g) and O2(g) can react to produce nitrogen monoxide, NO(g), as represented by the equation above.
(a) Write the expression for the equilibrium constant, Kp , for the forward reaction.
(b) A student injects N2(g) and O2(g) into a previously evacuated, rigid vessel and raises the temperature of the vessel to 2000°C. At this temperature the initial partial pressures of N2(g) and O2(g) are 6.01 atm and 1.61 atm, respectively. The system is allowed to reach equilibrium. The partial pressure of NO(g) at equilibrium is 0.122 atm. Calculate the value of Kp .
Nitrogen monoxide, NO(g), can undergo further reactions to produce acids such as HNO2 , a weak acid with a Ka of 4.0 × 10−4 and a pKa of 3.40.
(c) A student is asked to make a buffer solution with a pH of 3.40 by using 0.100 M HNO2(aq) and 0.100 M NaOH(aq).
(i) Explain why the addition of 0.100 M NaOH(aq) to 0.100 M HNO2(aq) can result in the formation of a buffer solution. Include the net ionic equation for the reaction that occurs when the student adds the NaOH(aq) to the HNO2(aq).
(ii) Determine the volume, in mL, of 0.100 M NaOH(aq) the student should add to 100. mL of 0.100 M HNO2(aq) to make a buffer solution with a pH of 3.40. Justify your answer.
(d) A second student makes a buffer by dissolving 0.100 mol of NaNO2(s) in 100. mL of 1.00 M HNO2(aq).
Which is more resistant to changes in pH when a strong acid or a strong base is added, the buffer made by the second student or the buffer made by the first student in part (c) ? Justify your answer.
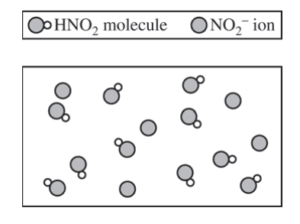
(e) A new buffer is made using HNO2(aq) as one of the ingredients. A particulate representation of a small representative portion of the buffer solution is shown below. (Cations and water molecules are not shown.) Is the pH of the buffer represented in the diagram greater than, less than, or equal to 3.40 ? Justify your answer.
▶️Answer/Explanation
Ans:
(a)
| \(K_{p}=\frac{(P_{NO})^{2}}{(P_{N_{2}})(P_{O_{2}})}\) |
(b)
N2(g) + O2(g) ⇔ 2 NO(g) 2x = 0.122 atm ⇒ x = 0.0610 atm \(K_{p}=\frac{(0.122)^{2}}{(5.95)(1.55)}=0.00161\) |
(c) (i)
NaOH will neutralize some of the HNO2 to produce NO2–. The resulting solution contains a mixture of a weak acid and its conjugate base, which is a buffer solution. HNO2 + OH– → NO2– + H2O |
(ii)
The student should add 50.0 mL of 0.100 M NaOH(aq). When half of the HNO2 is converted to the conjugate base, [HNO2] = [NO2– ], therefore the buffer has a pH equal to pKa. |
(d)
| The buffer made by the second student is more resistant to changes in pH because it contains a higher concentration of HNO2 and NO2– to react with added H+ or OH– ions. |
(e)
The pH of the solution is less than 3.40. If [HNO2] = [NO2– ], pH = pKa, and the pH of the solution would be 3.40. Since [HNO2] > [NO2– ], as represented in the diagram, the solution has a pH less than 3.40. |
Question
A student is given 50.0 mL of a solution of Na2CO3 of unknown concentration. To determine the concentration of the solution, the student mixes the solution with excess 1.0 M Ca(NO3)2(aq), causing a precipitate to form.
The balanced equation for the reaction is shown below.
Na2CO3(aq) + Ca(NO3)2(aq) → 2 NaNO3(aq) + CaCO3(s)
(a) Write the net ionic equation for the reaction that occurs when the solutions of Na2CO3 and Ca(NO3)2 are mixed.
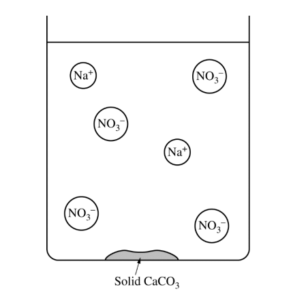
(b) The diagram below is incomplete. Draw in the species needed to accurately represent the major ionic species remaining in the solution after the reaction has been completed.
The student filters and dries the precipitate of CaCO3 (molar mass 100.1 g/mol) and records the data in the table below.

(c) Determine the number of moles of Na2CO3 in the original 50.0 mL of solution.
(d) The student realizes that the precipitate was not completely dried and claims that as a result, the calculated Na2CO3 molarity is too low. Do you agree with the student’s claim? Justify your answer.
(e) After the precipitate forms and is filtered, the liquid that passed through the filter is tested to see if it can conduct electricity. What would be observed? Justify your answer.
The student decides to determine the molarity of the same Na2CO3 solution using a second method. When
Na2CO3 is dissolved in water, CO32−(aq) hydrolyzes to form HCO3−(aq), as shown by the following equation.
CO32−(aq) + H2O(l) ⇔ HCO3−(aq) + OH−(aq) \(K_{b}=\frac{[HC{O_{3}}^{-}][OH^{-}]}{[{CO_{3}}^{2-}]}=2.1\times 10^{-4}\)
(f) The student decides to first determine [OH−] in the solution, then use that result to calculate the initial concentration of CO32−(aq).
(i) Identify a laboratory method (not titration) that the student could use to collect data to determine [OH−] in the solution.
(ii) Explain how the student could use the measured value in part (f)(i) to calculate the initial concentration of CO32−(aq). (Do not do any numerical calculations.)
(g) In the original Na2CO3 solution at equilibrium, is the concentration of HCO3−(aq) greater than, less than, or equal to the concentration of CO32−(aq) ? Justify your answer.
(h) The student needs to make a CO32−/HCO3− buffer. Is the Na2CO3 solution suitable for making a buffer with a pH of 6? Explain why or why not.
▶️Answer/Explanation
Ans:
(a)
| Ca2+(aq) + CO32-(aq) → CaCO3(s) |
(b)
| The drawing shows one Ca2+ ion. |
(c)
\(0.93 g CaCO_{3}\times \frac{1 mol CaCO_{3}}{100.1 g}=0.0093 mol CaCO_{3}\) \(0.0093 mol CaCO_{3}\times \frac{1 mol Na_{2}CO_{3}}{1 molCaCO_{3} }=0.0093 mol Na_{2}CO_{3}\) |
(d)
| Disagree. The presence of water in the solid will cause the measured mass of the precipitate to be greater than the actual mass of CaCO3. As a result, the calculated number of moles of CaCO3 and moles of Na2CO3 will be greater than the actual moles present. Therefore the calculated concentration of Na2CO3(aq) will be too high. |
(e)
| The liquid conducts electricity because ions (Na+(aq), Ca2+(aq), and NO3–(aq) are present in the solution. |
(f) (i)
| Determine the pH of the solution using a pH meter. |
(ii)
First determine [OH–] using pOH = 14 – pH, then [OH–] = 10-pOH. Then, use the Kb expression and an ICE table (see example below to determine [CO32-] and [HCO3–] at equilibrium. The initial concentration of CO32-, ci, is equal to the sum of the equilibrium concentrations of CO32- and HCO3–.
|
(g)
| Less than. The small value of Kb, 2.1 × 10-4, indicates that the reactants are favored. |
(h)
| No, the Na2CO3 solution is not suitable. The pKa of HCO3– is 10.32. Buffers are effective when the required pH is approximately equal to the pKa of the weak acid. An acid with a pKa of 10.32 is not appropriate to prepare a buffer with a pH of 6. |