Question
\(3Ag(s)+4HNO_{3}(aq)\rightarrow 3AgNO_{3}(aq)+NO(g)+2H_{2}O(l)\)
A student investigates the reaction between Ag(s) and \(HNO_3\)(aq) represented by the equation above.
(a) Predict the sign of the entropy change, ΔS°, for the reaction. Justify your answer.
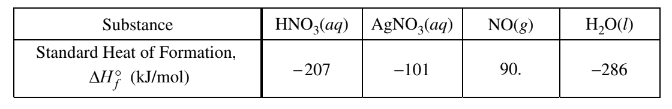
(b) Use the information in the table below to calculate the value of \(\Delta H^{\circ}_{rxn}\), the standard enthalpy change for the reaction, in\( kJ/mol_{rxn}\) .
(c) Based on your answers to parts (a) and (b), is the reaction more likely to be thermodynamically favorable at 25°C, or at 95°C? Justify your answer.
(d) The student runs the reaction using a 3 to 4 mole ratio of Ag(s) to\( HNO_3\)(aq). Suggest a method the student can use to isolate solid \(AgNO_3 \)from the other products of the reaction.
▶️Answer/Explanation
(a) The entropy change is positive because the reaction has one mole of gas in the products and none in the reactants.
(b)\( \Delta H^{\circ}_{rxn} = 3(-101) + 90. +2(-286) -4(-207)
= 43 kJ/molrxn\)
(c) \(\Delta G^{\circ}=\Delta H^{\circ}-T\Delta S^{\circ}\) The reaction is more likely to be favorable at \(95G^{\circ}\). At the higher temperature, the term\( T\Delta S^{\circ}\) is larger and positive; thus, when subtracted from \(\Delta H^{\circ}\), the value of \(\Delta G^{\circ} \) is more likely to be negative.
(d) The student can evaporate the water, leaving behind solid silver nitrate.
Question
Explain the difference between an amorphous and a crystalline solid on the microscopic level.
▶️Answer/Explanation
Ans:
Amorphous solids lack long-range order that is found in crystalline solids.
Amorphous: disordered structure. Crystalline: regular, repeating array of ions or atoms.
