Question
When a small amount of 12 M\( HNO_{3}\)(aq) is added to a buffer solution made by mixing \(CH_{ 3}NH_{2}\)(aq) and \(CH_{3}NH_{3}\)Cl(aq), the pH of the buffer solution changes from 10.64 to 10.62. Which of the following equations represents thereaction that accounts for the fact that the pH does not change significantly when the\( HNO_{3}\)(aq) is added?
(A)\( CH_{3}NH_{2}(aq) + H^{+}(aq) \)→ \(CH_{3}NH^{3+}(aq)\)
(B) \(CH_{3}NH_{3}^{+}(aq) + H^{+}(aq)\) → \(CH_{3}NH_{4} ^{2+}(aq)\)
(C) \(NO_{3} (aq) + H^{+}(aq)\) → \(HNO_{3}\)(aq)
(D) \(OH ^{-}(aq) + H^{+}(aq) \)→ \(H_{2}O\)(l)
▶️Answer/Explanation
Ans:A
The given scenario involves the addition of a small amount of \( HNO_{3} \) to a buffer solution made of \( CH_{3}NH_{2} \) and \( CH_{3}NH_{3}Cl \), resulting in a minimal change in pH. This indicates that the buffer system effectively resists changes in pH by neutralizing the added \( H^{+} \) ions.
The buffer capacity primarily relies on the presence of weak acid-base pairs that can react with added \( H^{+} \) or \( OH^{-} \) ions to maintain the pH.
(A) \( CH_{3}NH_{2}(aq) + H^{+}(aq) \rightarrow CH_{3}NH_{3}^{+}(aq) \) represents the reaction of the weak base \( CH_{3}NH_{2} \) with \( H^{+} \), which is consistent with the behavior of a buffer system.
(B) \( CH_{3}NH_{3}^{+}(aq) + H^{+}(aq) \rightarrow CH_{3}NH_{4} ^{2+}(aq) \) involves the protonation of the conjugate acid, which doesn’t explain the buffer action in this case.
(C) \( NO_{3} (aq) + H^{+}(aq) \rightarrow HNO_{3}(aq) \) represents the dissociation of the \( HNO_{3} \) added, which doesn’t explain the buffer action.
(D) \( OH ^{-}(aq) + H^{+}(aq) \rightarrow H_{2}O(l) \) represents neutralization, which occurs in the buffer solution but isn’t the primary mechanism by which a buffer resists changes in pH.
Among these options, (A) \( CH_{3}NH_{2}(aq) + H^{+}(aq) \rightarrow CH_{3}NH_{3}^{+}(aq) \) best represents the reaction that accounts for the minimal change in pH when \( HNO_{3} \) is added to the buffer solution.
So, the correct answer is:(A) \( CH_{3}NH_{2}(aq) + H^{+}(aq) \rightarrow CH_{3}NH_{3}^{+}(aq) \)
Question
To prepare a buffer solution for an experiment, a student measured out 53.49g of \(NH_4Cl(s)\) (molar mass 53.49g/mol) and added it to 1.0L of 1.0M \(NH_3(aq)\). However, in the process of adding the \(NH_4Cl(s)\) to the \(NH_3(aq)\), the student spilled some of the \(NH_4Cl(s)\) onto the bench top. As a result, only about 50.g of \(NH_4Cl(s)\) was actually added to the 1.0M \(NH_3(aq)\). Which of the following best describes how the buffer capacity of the solution is affected as a result of the spill?
A The solution has a greater buffer capacity for the addition of base than for acid, because \([NH_3]<[NH_4^+]\) .
B The solution has a greater buffer capacity for the addition of base than for acid, because \([NH_3]>[NH_4^+]\).
C The solution has a greater buffer capacity for the addition of acid than for base, because \([NH_3]<[NH_4^+]\).
D The solution has a greater buffer capacity for the addition of acid than for base, because \([NH_3]>[NH_4^+]\).
▶️Answer/Explanation
Ans:D
As a result of the spill, less than one mole of \(NH_4Cl(s)\) was added to the \(NH_3(aq)\), so \([NH_4^+]<1.0M\), which is the molarity of \(NH_3(aq)\). Because the buffer solution has more conjugate base than acid, the solution has a greater buffer capacity for the addition of acid than for base.
Question
\(HC_3H_5O_2(aq)+H_2O(l\))⇄\(H_3O^+(aq)+C_3H_5O_2^−(aq)\) \(pK_a=4.87\)
The acid ionization equilibrium for \(HC_3H_5O_2(aq)\) is represented by the equation above. A mixture of 1.00L of 0.100M \(HC_3H_5O_2(aq)\) and 0.500L of 0.100M \(NaOH\) will produce a buffer solution with a \(pH=4.87\). If the \(NaOH\) solution was mislabeled and was \1.00M instead of 0.100M, which of the following would be true?
A The \(pH\) of the resulting solution would still be 4.87 because buffer solutions regulate changes in pH regardless of how much NaOH is added.
B The \(pH\) of the resulting solution would be somewhat higher than 4.87 because adding NaOH that is 10 times more concentrated makes the concentration of the conjugate base 10 times larger.
C The \(pH\) of the resulting solution would be much higher than 4.87 because the weak acid would be completely neutralized by the larger amount of NaOH added.
D The \(pH\) of the resulting solution would still be 4.87 because the solution contains a large volume of the weak acid to react with the NaOH and would maintain the same pH .
▶️Answer/Explanation
Ans:C
The weak acid is completely neutralized by adding 0.500L of 1.00M \(NaOH\) to 1.00L of 0.100M \(HC_3H_5O_2\) according to the reaction: \(HC_3H_5O_2(aq)+OH^−(aq)\)⇄\(C_3H_5O_2^−(aq)+H_2O(l)\). The resulting solution will not be a buffer because there is no conjugate acid-base pair left in solution, and the pH would drastically increase due to the excess amount of \(OH^−\) ions in solution.
Question
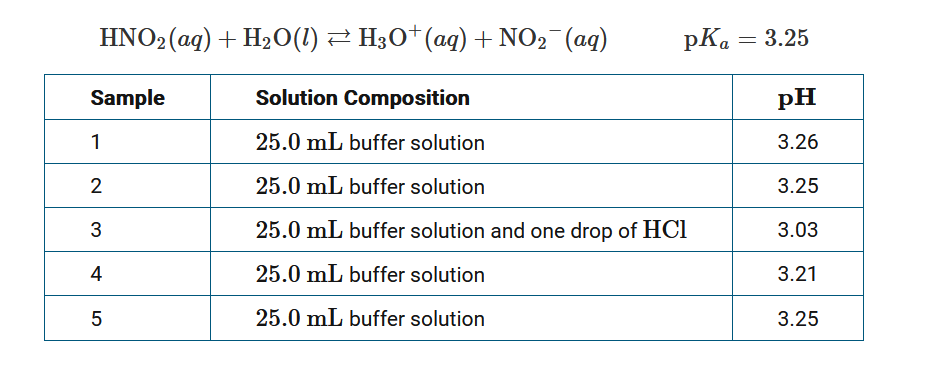
The acid ionization equilibrium for \(HNO_2\) is represented by the equation above. A 250.0mL buffer solution is prepared by mixing 125.0mL of 0.20M \(HNO_2\) and 125.0mL of 0.10M \(NaOH\). To test the buffer capacity, the \(pH\) is measured and recorded in the table for four samples of the buffer and one sample of a mixture of the buffer and \(HCl\). Which of the following best helps explain why the \(pH\) of sample 4 is lower than the \(pH\) of the other samples containing only buffer solution?
A Prior to measuring the \(pH\)of sample 4, some water evaporated, resulting in an increase in the concentration of \(HNO_2\) in the sample.
B After measuring the \(pH\)of the more acidic sample 3, the \(pH\) probe was not rinsed and wiped, resulting in the neutralization of a very small amount of the conjugate base in sample 4.
C Prior to measuring the \(pH\)of sample 4, the room temperature dropped suddenly, resulting in an increase in the \pK_a\) for \(HNO_2\) .
D The volume used to measure the \(pH\)of sample 4 was less than 25.0mL, resulting in a decrease in the concentration of \(NO_2^−\) .
▶️Answer/Explanation
Ans:B
The best explanation for the lower \(pH\)reading is that the p\(pH\) probe was not rinsed and wiped prior to measuring the \(pH\) of sample 4, and the residue from the more acidic sample 3 lowered the \(pH\) of sample 4 by neutralizing a very small amount of \(NO_2^−\).
Question
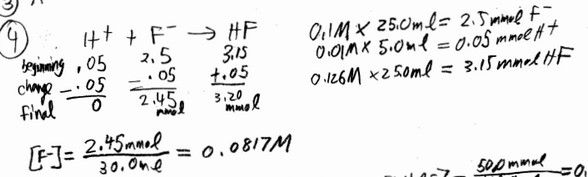
Consider a solution containing 0.100 M fluoride ions and 0.126 M hydrogen fluoride. The concentration of
fluoride ions after the addition of 5.00 mL of 0.0100 M HCl to 25.0 mL of this solution is __________ M.
A) 0.00167 B) 0.0817 C) 0.00253 D) 0.0850 E) 0.0980
▶️Answer/Explanation
Ans: B