Question

Some binary compounds that form between fluorine and various nonmetals are listed in the table above. A student examines the data in the table and poses the following hypothesis: the number of F atoms that will bond to a nonmetal is always equal to 8 minus the number of valence electrons in the nonmetal atom.
(a) Based on the student’s hypothesis, what should be the formula of the compound that forms between chlorine and fluorine?
(b) In an attempt to verify the hypothesis, the student researches the fluoride compounds of the other halogens and finds the formula \(CIF_{3}\). In the box below, draw a complete Lewis electron-dot diagram for a molecule of\( CIF_{3}\).

(c) Two possible geometric shapes for the\( CIF_{3}\) molecule are trigonal planar and T-shaped. The student does some research and learns that the molecule has a dipole moment. Which of the two shapes is consistent with the fact that the \( CIF_{3}\) molecule has a dipole moment? Justify your answer in terms of bond polarity and molecular structure.
In an attempt to resolve the existence of the \( CIF_{3}\) molecule with the hypothesis stated above, the student researches the compounds that form between halogens and fluorine, and assembles the following list.
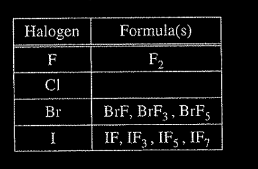
(d) Based on concepts of atomic structure and periodicity, propose a modification to the student’s previous hypothesis to account for the compounds that form between halogens and fluorine.
▶️Answer/Explanation
(a) \(CLF\)
(b) 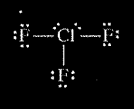
(c) The molecule is T-shaped because a T-shaped structure is asymmetric with dipoles that do not cancel out, but produce a net dipole (i.e., a polar molecule).
OR
because, if the molecule had a trigonal planar structure, the molecule would be symmetric with dipoles that cancel out and produce a net dipole of zero (i.e., a nonpolar molecule), which is not consistent with the observation that the CIF3 molecule does have a dipole moment.
(d) An acceptable hypothesis (descriptive or formulaic) must include the following ideas:
1. Atomic Structure; e.g., odd number of F atoms
2. Periodicity: e.g., as the atomic number of the central halogen atom increases, the number of F atoms increases.
Question
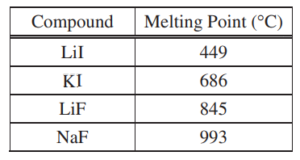
A student learns that ionic compounds have significant covalent character when a cation has a polarizing effect on a large anion. As a result, the student hypothesizes that salts composed of small cations and large anions should have relatively low melting points.
(a) Select two compounds from the table and explain how the data support the student’s hypothesis.
(b) Identify a compound from the table that can be dissolved in water to produce a basic solution. Write the net ionic equation for the reaction that occurs to cause the solution to be basic.
▶️Answer/Explanation
Ans:
(a)
Lil and KI. Lil has a small cation and a large anion and KI has a large cation and the same large anion. The melting point of LiI (with its smaller cation) is lower than that of KI. OR LiI and LiF. LiI has a small cation and a large anion and LiF has the same small cation and a small anion. The melting point of LiI (with its larger anion) is lower than that of LiF. OR LiI and NaF. LiI has a small cation and a large anion and NaF has a relatively small cation and a small anion. The melting point of LiI (with its larger anion) is lower than that of NaF. |
(b)
Either LiF or NaF is acceptable. F– + H2O ⇔ HF + OH– |
Question
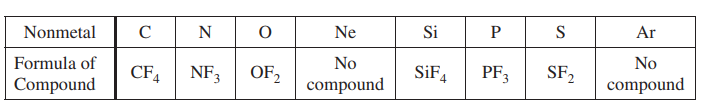
Some binary compounds that form between fluorine and various nonmetals are listed in the table above. A student examines the data in the table and poses the following hypothesis: the number of F atoms that will bond to a nonmetal is always equal to 8 minus the number of valence electrons in the nonmetal atom.
(a) Based on the student’s hypothesis, what should be the formula of the compound that forms between chlorine and fluorine?
(b) In an attempt to verify the hypothesis, the student researches the fluoride compounds of the other halogens and finds the formula ClF3 . In the box below, draw a complete Lewis electron-dot diagram for a molecule of ClF3 .
(c) Two possible geometric shapes for the ClF3 molecule are trigonal planar and T-shaped. The student does some research and learns that the molecule has a dipole moment. Which of the two shapes is consistent with the fact that the ClF3 molecule has a dipole moment? Justify your answer in terms of bond polarity and molecular structure.
In an attempt to resolve the existence of the ClF3 molecule with the hypothesis stated above, the student researches the compounds that form between halogens and fluorine, and assembles the following list.
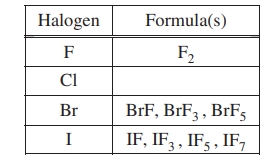
(d) Based on concepts of atomic structure and periodicity, propose a modification to the student’s previous hypothesis to account for the compounds that form between halogens and fluorine.
▶️Answer/Explanation
Ans:
(a)
| CIF |
(b)
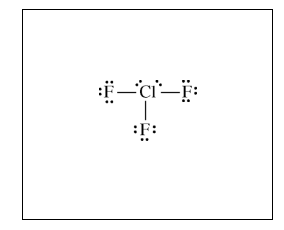
| See diagram above |
(c)
| The molecule is T-shaped because a T-shaped structure is asymmetric with dipoles that do not cancel out, but produce a net dipole (i.e., a polar molecule). OR because, if the molecule had a trigonal planar structure, the molecule would be symmetric with dipoles that cancel out and produce a net dipole of zero (i.e., a nonpolar molecule), which is not consistent with the observation that the ClF3 molecule does have a dipole moment. |
(d)
| An acceptable hypothesis (descriptive or formulaic) must include the following ideas: 1. Atomic Structure: e.g., odd number of F atoms 2. Periodicity: e.g., as the atomic number of the central halogen atom increases, the number of F atoms increases. |
