Question
6 H+(aq) + 2 MnO4−(aq) + 5 H2C2O4(aq) → 10 CO2(g) + 8 H2O(l) + 2 Mn2+(aq)
A student dissolved a 0.139 g sample of oxalic acid, H2C2O4 , in water in an Erlenmeyer flask. Then the student titrated the H2C2O4 solution in the flask with a solution of KMnO4 , which has a dark purple color. The balanced chemical equation for the reaction that occurred during the titration is shown above.
(a) Identify the species that was reduced in the titration reaction. Justify your answer in terms of oxidation numbers.
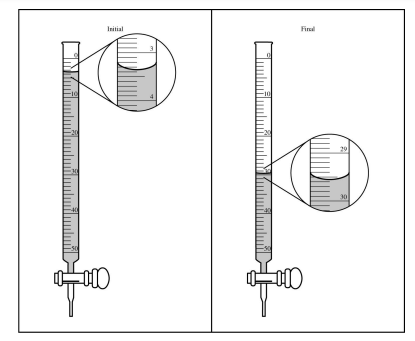
(b) The student used a 50.0 mL buret to add the KMnO4(aq) to the H2C2O4(aq) until a faint lavender color was observed in the flask, an indication that the end point of the titration had been reached. The initial and final volume readings of the solution in the buret are shown below. Write down the initial reading and the final reading and use them to determine the volume of KMnO4(aq) that was added during the titration
(c) Given that the concentration of KMnO4(aq) was 0.0235 M, calculate the number of moles of MnO4− ions that completely reacted with the H2C2O4 .
(d) The student proposes to perform another titration using a 0.139 g sample of H2C2O4 , but this time using 0.00143 M KMnO4(aq) in the buret. Would this titrant concentration be a reasonable choice to use if the student followed the same procedure and used the same equipment as before? Justify your response.
▶️Answer/Explanation
Ans:
(a)
| MnO4– is reduced to Mn2+ as the oxidation number of Mn changes from +7 to +2, indicating a gain of 5 electrons. |
(b)
| 29.55 mL – 3.35 mL = 26.20 mL |
(c)
| (0.02620 L) (0.0235 mol/L) = 0.000616 mol |
(d)
| No. The 0.00143 M titrant solution is so diluted that the volume of titrant needed to reach the end point would be much greater than the 50 mL capacity of the buret. |
Question
2 NO(g) + O2(g) → 2 NO2(g)
A student investigates the reactions of nitrogen oxides. One of the reactions in the investigation requires an equimolar mixture of NO(g) and NO2(g), which the student produces by using the reaction represented above.
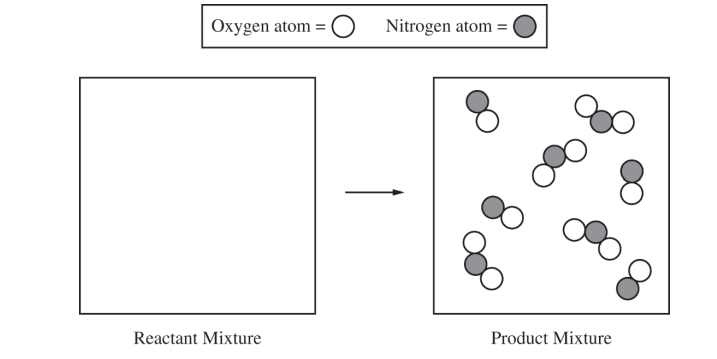
(a) The particle-level representation of the equimolar mixture of NO(g) and NO2(g) in the flask at the completion of the reaction between NO(g) and O2(g) is shown below in the box on the right. In the box below on the left, draw the particle-level representation of the reactant mixture of NO(g) and O2(g) that would yield the product mixture shown in the box on the right. In your drawing, represent oxygen atoms and nitrogen atoms as indicated below.
The student reads in a reference text that NO(g) and NO2(g) will react as represented by the equation below.
Thermodynamic data for the reaction are given in the table below the equation.
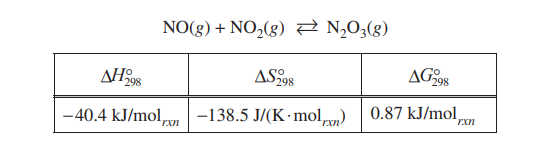
(b) The student begins with an equimolar mixture of NO(g) and NO2(g) in a rigid reaction vessel and the mixture reaches equilibrium at 298 K.
(i) Calculate the value of the equilibrium constant, K, for the reaction at 298 K.
(ii) If both PNO and PNO2 in the vessel are initially 1.0 atm, will PN2O3 at equilibrium be equal to 1.0 atm? Justify your answer.
(c) The student hypothesizes that increasing the temperature will increase the amount of N2O3(g) in the equilibrium mixture. Indicate whether you agree or disagree with the hypothesis. Justify your answer.
N2O3(g) reacts with water to form nitrous acid, HNO2 (aq), a compound involved in the production of acid rain.
The reaction is represented below.
N2O3(g) + H2O(l) → 2 HNO2(aq)
(d) The skeletal structure of the HNO2 molecule is shown in the box below.
(i) Complete the Lewis electron-dot diagram of the HNO2 molecule in the box below, including any lone pairs of electrons.

(ii) Based on your completed diagram above, identify the hybridization of the nitrogen atom in the HNO2 molecule. To produce an aqueous solution of HNO2 , the student bubbles N2O3(g) into distilled water. Assume that the reaction goes to completion and that HNO2 is the only species produced. To determine the concentration of HNO2(aq) in the resulting solution, the student titrates a 100. mL sample of the solution with 0.100 M KOH(aq).
The neutralization reaction is represented below.
HNO2(aq) + OH−(aq) → NO2−(aq) + H2O(l)
The following titration curve shows the change in pH of the solution during the titration.
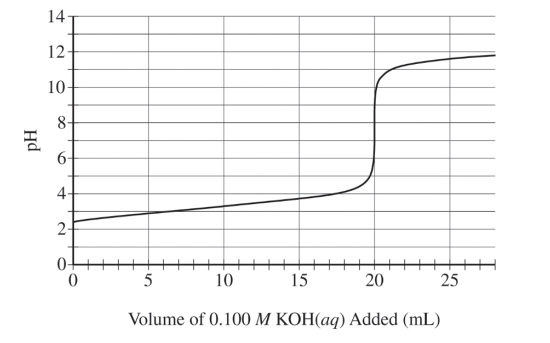
(e) Use the titration curve and the information above to
(i) determine the initial concentration of the HNO2(aq) solution
(ii) estimate the value of pKa for HNO2(aq)
(f) During the titration, after a volume of 15 mL of 0.100 M KOH(aq) has been added, which species, HNO2(aq) or NO2−(aq), is present at a higher concentration in the solution? Justify your answer.
▶️Answer/Explanation
Ans:
(a)
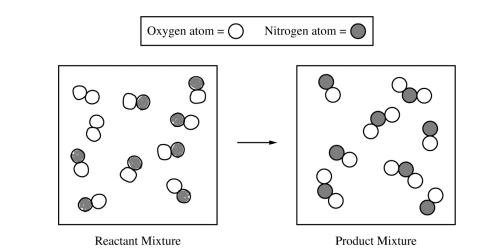
| See sample student response above. (8 molecules of NO and 2 molecules of O2) |
(b) (i)
ΔG0 = – RT In K \(K = e^{-\frac{870 J/mol}{(8.314 J mol^{-1}K^{-1})(298 K)}}\) |
(ii)
No, the pressure will not equal 1.0 atm. PN2O3 would only equal 1.0 atm if the reaction goes to completion. |
(c)
Disagree. Because the reaction is exothermic, increasing the temperature of the reaction will favor the formation of the reactants (according to Le Chatelier’s principle). |
(d)

(i)
| See sample response above. (Line segments can be used to represent electron pairs.) |
(ii)
| sp2 |
(e) (i)
20. mL \(KOH\times \frac{0.100 mol KOH}{1000 mL KOH}=0.0020 mol\) KOH added \(\frac{0.0020 mol HNO_{2}}{0.100 L}=0.020 M HNO_{2}\) |
(ii)
| The value of pKa is about 3.4. |
(f)
| NO2–(aq) The titration is past the half-equivalence point; therefore, there will be more conjugate base present than acid. |
Question
Answer the following questions relating to Fe and its ions, Fe2+ and Fe3+.
(a) Write the ground-state electron configuration of the Fe2+ ion.
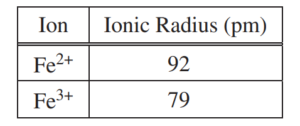
(b) The radii of the ions are given in the table above. Using principles of atomic structure, explain why the radius of the Fe2+ ion is larger than the radius of the Fe3+ ion.
(c) Fe3+ ions interact more strongly with water molecules in aqueous solution than Fe2+ ions do. Give one reason for this stronger interaction, and justify your answer using Coulomb’s law.
A student obtains a solution that contains an unknown concentration of Fe2+(aq). To determine the concentration
of Fe2+(aq) in the solution, the student titrates a sample of the solution with MnO4 –(aq) , which converts Fe2+(aq) to Fe3+(aq), as represented by the following equation.
5 Fe2+(aq) + MnO4−(aq) + 8 H+(aq) → 5 Fe3+(aq) + Mn2+(aq) + 4 H2O(l)
(d) Write the balanced equation for the half-reaction for the oxidation of Fe2+(aq) to Fe3+(aq).
(e) The student titrates a 10.0 mL sample of the Fe2+(aq) solution. Calculate the value of [Fe2+] in the solution if it takes 17.48 mL of added 0.0350 M KMnO4(aq) to reach the equivalence point of the titration.
To deliver the 10.0 mL sample of the Fe2+(aq) solution in part (e), the student has the choice of using one of the pieces of glassware listed below.
• 25 mL buret • 25 mL beaker
• 25 mL graduated cylinder • 25 mL volumetric flask
(f) Explain why the 25 mL volumetric flask would be a poor choice to use for delivering the required volume of the Fe2+(aq) solution.
In a separate experiment, the student is given a sample of powdered Fe(s) that contains an inert impurity. The student uses a procedure to oxidize the Fe(s) in the sample to Fe2O3(s). The student collects the following data during the experiment.
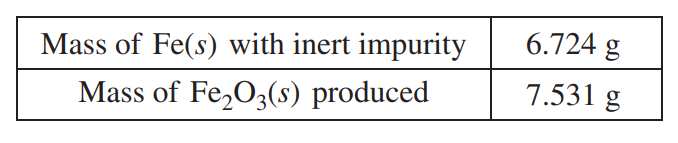
(g) Calculate the number of moles of Fe in the Fe2O3(s) produced.
(h) Calculate the percent by mass of Fe in the original sample of powdered Fe(s) with the inert impurity.
(i) If the oxidation of the Fe(s) in the original sample was incomplete so that some of the 7.531 g of product was FeO(s) instead of Fe2O3(s), would the calculated mass percent of Fe(s) in the original sample be higher, lower, or the same as the actual mass percent of Fe(s)? Justify your answer
▶️Answer/Explanation
Ans:
(a)
| 1s2 2s2 2p6 3s2 3p6 3d6 OR [Ar] 3d6 |
(b)
| Both ions have the same nuclear charge; however, the greater number of electrons in the outermost shell of Fe2+ results in greater electron – electron repulsion that shell, leading to a larger radius. |
(c)
| Coulomb’s law : \(F \propto \frac{q_{1}q_{2}}{r^{2}}\) (need not be explicitly stated) IN comparison to the Fe2+ ion, the Fe3+ ion has a higher charge. OR The smaller size of Fe3+ allows it to get closer to a water molecule. |
(d)
| Fe2+(aq) → Fe3+ (aq) + e– |
(e)
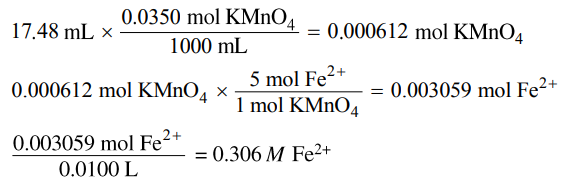 |
(f)
| The volumetric flask is designed to contain only 25.00 mL precisely. |
(g)
7.531 g Fe2O3 × \(\frac{1 mol Fe_{2}O_{3}}{159.70 g Fe_{2}O_{3}}\) = 0.04716 mol Fe2O3 0.04716 mol Fe2O3 × \(\frac{2 mol Fe}{1 mol Fe_{2}O_{3}}\) = 0.09431 mol Fe |
(h)
0.09431 mol Fe × \(\) = 5.267 g Fe \(\frac{5.267 g Fe}{6.724 g Sample}\times 100= 78.33%\) |
(i)
The calculated mass percent of Fe would be lower than the actual mass percent of Fe. A sample that contains any FeO (rather than Fe2O3) will have a higher actual mass percent of Fe than a completely oxidized sample would have. Therefore, when the moles of Fe are calculated (assuming all the mass of the sample is Fe2O3) the calculated number of moles of Fe, and hence the calculated mass percent of Fe, will be lower. |
