Questions
After a certain pesticide compound is applied to crops, its decomposition is a first-order reaction with a half-life of 56 days. What is the rate constant, k, for the decomposition reaction?
(A) \(0.012 day^{−1}\)
(B) \(0.018 day^{−1}\)
(C)\( 56 day^{−1}\)
(D) \(81 day^{−1}\)
▶️Answer/Explanation
Ans: D
The half-life (\( t_{1/2} \)) of a first-order reaction is related to the rate constant (\( k \)) by the equation:
\[ t_{1/2} = \frac{0.693}{k} \]
Given that the half-life (\( t_{1/2} \)) is 56 days, we can use this equation to solve for the rate constant (\( k \)):
\[ 56 = \frac{0.693}{k} \]
\[ k = \frac{0.693}{56} \]
\[ k \approx 0.012 \, \text{day}^{-1} \]
So, the correct answer is:
(A) \( 0.012 \, \text{day}^{-1} \)
Question
\(2 NO_2(g) → N_2O_4\) (g)
dark brown colorless
The dimerization of NO2(g), an exothermic process, is represented by the equation above.
A 0.0300 mol sample of \(NO_2\)(g) is placed in a rigid 1.00 L reaction vessel and allowed to reach equilibrium at a certain temperature. What is the value of Kc at this temperature if 0.00500 mol of \(N_2O_4\)(g) is present at equilibrium?
(A) 0.0800
(B) 0.250
(C) 4.00
(D) 12.5
▶️Answer/Explanation
Ans:D
The equilibrium constant \(K_c\) is given by the ratio of the concentrations of the products to the reactants, each raised to the power of their stoichiometric coefficients. For the reaction:
\(2 NO_2(g) \rightarrow N_2O_4(g)\)
The equilibrium constant expression is:
\(K_c = \frac{[N_2O_4]}{[NO_2]^2}\)
Given that 0.0300 mol of \(NO_2\) is initially present in a 1.00 L vessel, the initial concentration of \(NO_2\) is 0.0300 mol/L. At equilibrium, 0.00500 mol of \(N_2O_4\) is present, so the equilibrium concentration of (N_2O_4) is 0.00500 mol/L.
Since the reaction consumes 2 moles of \(NO_2\) for every mole of \(N_2O_4\) produced, the equilibrium concentration of \(NO_2\) is the initial concentration minus twice the equilibrium concentration of \(N_2O_4\), which is $0.0300 mol/L – 2\times 0.00500 mol/L = 0.0200 mol/L$.
Substituting these values into the equilibrium constant expression gives:
\(K_c = \frac{0.00500}{(0.0200)^2} = 12.5\)
Question

Equimolar samples of \(CH _3OH(l)\) and \(C_2H_5OH(l)\) are placed in separate, previously evacuated, rigid 2.0 L vessels. Each vessel is attached to a pressure gauge, and the temperatures are kept at 300 K. In both vessels, liquid is observed to remain present at the bottom of the container at all times. The change in pressure inside the vessel containing \(CH_3OH(l) \)is shown below.

The value of\( K_p \) for the evaporation of \(CH_{3}\)OH(l) at 300 K is closest to
(A) 0.04
(B) 0.2
(C) 5
(D) 30
▶️Answer/Explanation
Ans:A
To solve this problem completely, we can first determine the number of moles of \(CH_3OH(g)\) present at equilibrium using the ideal gas law:
\[ PV = nRT \]
We rearrange this equation to solve for \( n \):
\[ n = \frac{PV}{RT} \]
Given:
\( P = 0.196 \, \text{atm} \)
\( V = 2.0 \, \text{L} \)
\( T = 300 \, \text{K} \)
\( R = 0.0821 \, \text{atm} \cdot \text{L/mol} \cdot \text{K} \)
\[ n = \frac{(0.196 \, \text{atm}) \times (2.0 \, \text{L})}{(0.0821 \, \text{atm} \cdot \text{L/mol} \cdot \text{K}) \times (300 \, \text{K})} \]
\[ n = \frac{0.392 \, \text{mol} \cdot \text{atm}}{24.63 \, \text{L}} \]
\[ n ≈ 0.016 \, \text{mol} \]
Now, \( n \) represents the number of moles of \( CH_3OH(g) \) present at equilibrium.
The equilibrium constant \( K_p \) for the evaporation of \( CH_3OH(l) \) can be expressed as:
\[ K_p = \frac{{P_{CH_3OH}}}{{P_0}} \]
Where:
\( P_{CH_3OH} \) is the partial pressure of \( CH_3OH(g) \) in the equilibrium mixture.
\( P_0 \) is the standard pressure, which we can assume to be \( 1 \, \text{atm} \).
We’ve found that \( n \) is approximately \( 0.0159 \, \text{mol} \). To find \( P_{CH_3OH} \), we can use:
\[ P_{CH_3OH} = \frac{nRT}{V} \]
Substituting the values:
\[ P_{CH_3OH} = \frac{(0.0159 \, \text{mol}) \times (0.0821 \, \text{atm} \cdot \text{L/mol} \cdot \text{K}) \times (300 \, \text{K})}{2.0 \, \text{L}} \]
\[ P_{CH_3OH} ≈ \frac{0.392 \, \text{atm} \cdot \text{mol}}{2.0 \, \text{L}} \]
\[ P_{CH_3OH} ≈ 0.196 \, \text{atm} \]
Now, we can calculate \( K_p \):
\[ K_p = \frac{{0.196 \, \text{atm}}}{{1 \, \text{atm}}} \]
\[ K_p = 0.196 \]
Since \( K_p \) is closest to one of the provided options, we find that the closest option to \( 0.196 \) is \( (A) \) \( 0.04 \).
So, the answer is \( (A) \) \( 0.04 \).
Question

Equimolar samples of \(CH _3OH(l)\) and \(C_2H_5OH(l)\) are placed in separate, previously evacuated, rigid 2.0 L vessels. Each vessel is attached to a pressure gauge, and the temperatures are kept at 300 K. In both vessels, liquid is observed to remain present at the bottom of the container at all times. The change in pressure inside the vessel containing \(CH_3OH(l) \)is shown below.

The temperature of the \(CH_3OH\) is increased from 300 K to 400 K to vaporize all the liquid, which increases the pressure in the vessel to 0.30 atm. The experiment is repeated under identical conditions but this time using half the mass of\(CH_{3}\)OH that was used originally. What will be the pressure in the vessel at 400 K ?
(A) 0.15 atm
(B) 0.30 atm
(C) 0.40 atm
(D) 0.60 atm
▶️Answer/Explanation
Ans:A
First, let’s find the number of moles of \( CH_{3}OH(g) \) at 300 K using the initial conditions.
Given:
– Initial temperature (\( T_1 \)) = 300 K
– Initial pressure (\( P_1 \)) = 0.196 atm
– Initial volume (\( V_1 \)) = 2.0 L
We previously found that the number of moles (\( n_1 \)) at 300 K is approximately 0.0159 mol.
Now, let’s find the number of moles of \( CH_{3}OH(g) \) at 400 K using the final conditions.
Given:
– Final temperature (\( T_2 \)) = 400 K
– Final pressure (\( P_2 \)) = 0.30 atm
To find the number of moles (\( n_2 \)) at 400 K, we’ll use the ideal gas law:
\[ n_2 = \frac{{P_2 \cdot V_1}}{{R \cdot T_2}} \]
Where:
– \( P_2 \) is the final pressure (0.30 atm)
– \( V_1 \) is the initial volume (2.0 L)
– \( R \) is the ideal gas constant (0.0821 atm L / mol K)
– \( T_2 \) is the final temperature (400 K)
Substituting the values:
\[ n_2 = \frac{{0.30 \, \text{atm} \times 2.0 \, \text{L}}}{{0.0821 \, \text{atm} \cdot \text{L/mol} \cdot \text{K} \times 400 \, \text{K}}} \]
\[ n_2 ≈ \frac{{0.60 \, \text{mol} \cdot \text{atm}}}{{32.84 \, \text{L}}} \]
\[ n_2 ≈ 0.0183 \, \text{mol} \]
Now, since the experiment is repeated with half the mass of \( CH_{3}OH \), the number of moles of \( CH_{3}OH(g) \) at 400 K will also be halved. Thus, \( n_2 = 0.0183 \, \text{mol} \times 0.5 = 0.00915 \, \text{mol} \).
Finally, we can use the ideal gas law to find the pressure (\( P_3 \)) in the vessel at 400 K with half the initial mass:
\[ P_3 = \frac{{n_2 \cdot R \cdot T_2}}{{V_1}} \]
Substituting the values:
\[ P_3 = \frac{{0.00915 \, \text{mol} \times 0.0821 \, \text{atm} \cdot \text{L/mol} \cdot \text{K} \times 400 \, \text{K}}}{{2.0 \, \text{L}}} \]
\[ P_3 ≈ \frac{{0.3014 \, \text{atm} \cdot \text{mol}}}{{2.0 \, \text{L}}} \]
\[ P_3 ≈ 0.1507 \, \text{atm} \]
So, the pressure in the vessel at 400 K when using half the mass of \( CH_{3}OH \) will be approximately \( 0.15 \, \text{atm} \).
Therefore, the answer is (A) \( 0.15 \, \text{atm} \).
Question
\(2N_2O_5(g)→4NO_2(g)+O_2(g)\)
For the reaction represented by the equation above, the concentration of \(N_2O_5\) was measured over time. The following graphs were created using the data.
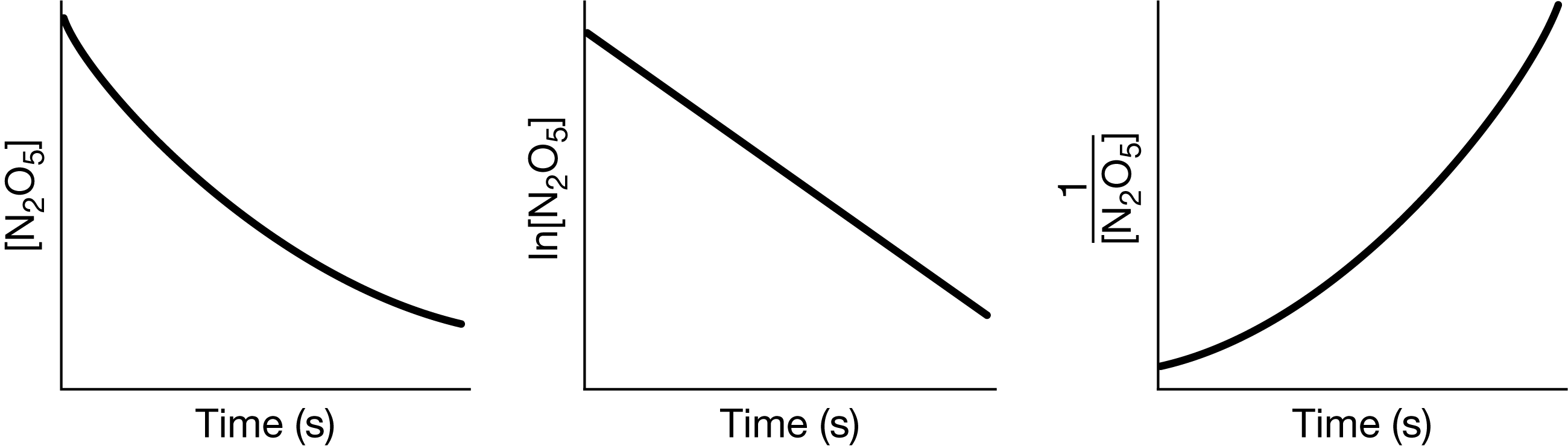
For the reaction represented by the equation above, the concentration of \(N_2O_5\) was measured over time. The following graphs were created using the data. Based on the graphs above, what is the order of the reaction with respect to \(N_2O_5\)?
A Zeroth order
B First order
C Second order
D Third order
▶️Answer/Explanation
Ans:B
For a first-order reaction, graphing \(ln[N_2O_5]\) versus time produces a straight line.
Question
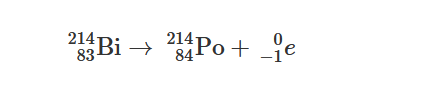
Bismuth-214 undergoes first-order radioactive decay to polonium-214 by the release of a beta particle, as represented by the nuclear equation above. Which of the following quantities plotted versus time will produce a straight line? (n = the number of moles in the sample)
A \(n_{Bi}\)
B \(n_{Po}\)
C \(ln(n_{Bi})\)
D \(\frac{1}{n_{Bi}}\)
▶️Answer/Explanation
Ans:C
Because the reaction is a first-order radioactive decay, a plot of \(n_{Bi}\) versus time would produce a straight line.
Question
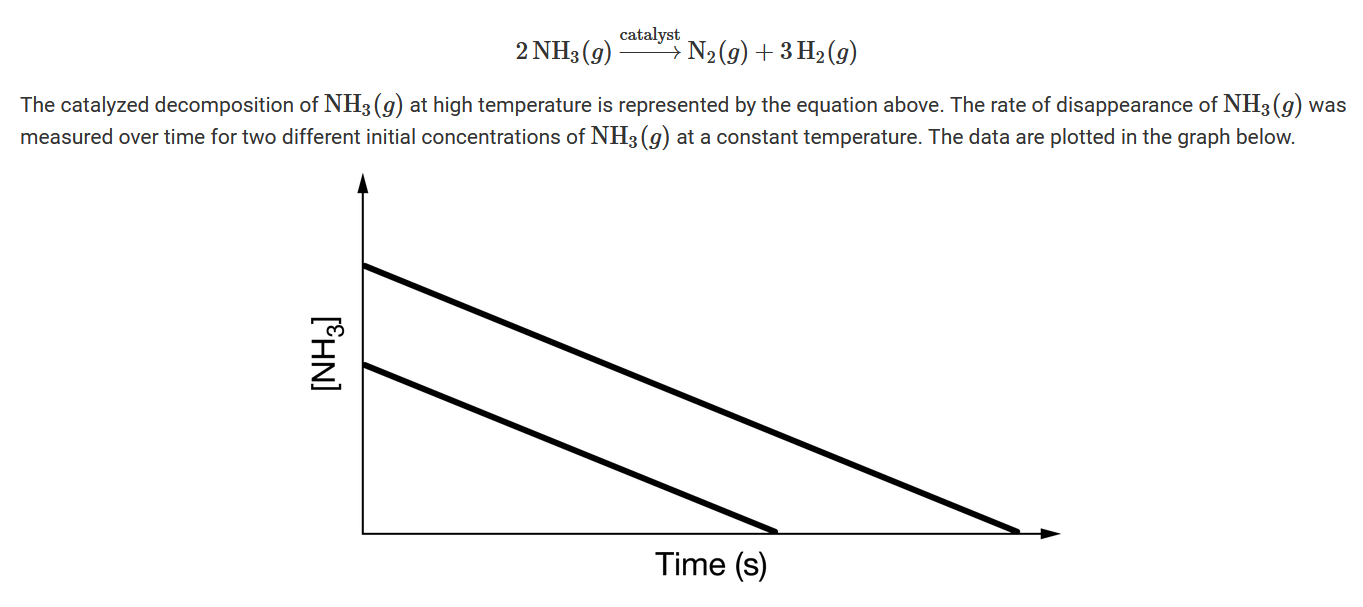
On the basis of the data in the graph, which of the following best represents the rate law for the catalyzed decomposition of \(NH_3\)(g)?
A Rate\(=k\)
B Rate\(=k[NH_3]\)
C Rate\(=k[NH_3]^2\)
D Rate\(=k[N_2][H_2]\)
▶️Answer/Explanation
Ans:A
The plots of \([NH_3]\) versus time for the two different initial concentrations of ammonia are straight lines with identical slopes. The slope is equal to \(\frac{\Delta[NH3]}{\Delta t}\), which is constant and equal to the rate of reaction. In other words, the rate is independent of \([NH_3]\), and the reaction is zeroth order with rate=k.
Refer to the following.
\(5 H_2O_2(aq) + 2 MnO_{4} ̄(aq) + 6 H^{+}(aq) \rightarrow 2 Mn^{2+}(aq) + 8 H_{2}O(l) + 5 O_{2}(g)\)
In a titration experiment,\( H_{2}O_{2}(aq)\) reacts with aqueous\( MnO_4^{+}(aq) \)as represented by the equation above. The dark purple \(KMnO_{4}\) solution is added from a buret to a colorless, acidified solution of\( H_{2}O_{2}\)(aq) in an Erlenmeyer flask. (Note: At the end point of the titration, the solution is a pale pink color.)
Question
At a certain time during the titration, the rate of appearance of \(O_2\)(g) was \(1.0 × 10^{-3}\) mol/(L·s). What was the rate of disappearance of \(MnO_4^{-}\) at the same time?
(A) 6.0 ×\( 10^{-3}\) mol/(L.s)
(B) 4.0 ×\( 10^{-3}\) mol/(L.s)
(C)\( 6.0 × 10^{-4}\) mol/(L.s)
(D) 4.0 x 10 mol/(L.s)
▶️Answer/Explanation
Ans:D