Question
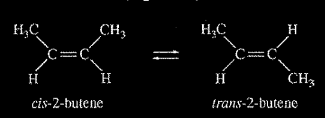
The half-life (1/2) of the catalyzed isomerization of cis-2-butene gas to produce trans-2-butene gas, represented above, was measured under various conditions, as shown in the table below.
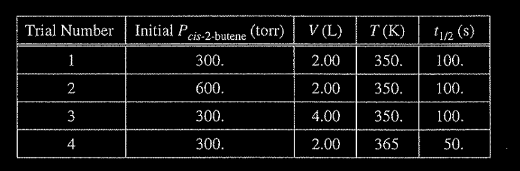
(a) The reaction is first order. Explain how the data in the table are consistent with a first-order reaction.
(b) Calculate the rate constant, k, for the reaction at 350. K. Include appropriate units with your answer.
(c) Is the initial rate of the reaction in trial I greater than, less than, or equal to the initial rate in trial 2? Justify your answer.
(d) The half-life of the reaction in trial 4 is less than the half-life in trial 1. Explain why, in terms of activation energy.
▶️Answer/Explanation
(a) For a first-order reaction, the half-life is independent of reactant concentration (or pressure) at constant 7, as shown in trials 1, 2, and 3.
(b) \(k=\frac{0.693}{t_{1/2}}=\frac{ 0.693}{ 100 s} = 0.00693 s^{-1}\)
(c) The initial rate in trial I is less than that in trial 2 because rate = k[cis-2-butene] or rate \(= k _{Pcis-2-butene}\) (with reference to values from both trials). OR because the initial concentration of cis-2-butene in trial 1 is less than that in trial 2 and it is constant.
(d) The temperature is higher in trial 4, meaning that the \(KE_{avg}\) of the molecules is greater. Consequently, in this trial a greater fraction of collisions have sufficient energy to overcome the activation energy barrier, thus the rate is greater.
Question.
\(H_{3}BO_{3}(aq)+4HF(g)\rightarrow H_{3}O^{+}(aq)+BF_{4}^{-}(aq)+2H_{2}O(l)\)
Tetrafluoroboric acid is a strong acid with the formula\( HBF_4\). The acid can be prepared by reacting the weak acid \(H_3BO_3\) (molar mass 61.83 g/mol) with HF according to the equation above.
(a) To prepare a solution of \(BF_4^{-}\)(aq), HF(g) is bubbled into a solution containing 50.0 g of\( H_3BO_3\) in a 1 L reaction vessel.
(i) Calculate the maximum number of moles of\( BF_4^{-}\)(aq) that can be produced.
(ii) Calculate the number of liters of HF(g), measured at 273 K and 1.00 atm, that will be consumed if all the\( H_3BO_3\) reacts.
(iii) Will the pH of the solution increase, decrease, or remain the same during the course of the reaction? Justify your answer. In another experiment, a 0.150 M \(BF_4^-\)(aq) solution is prepared by dissolving \(NaBF_4\)(s) in distilled water. The\( BF_{4}^-\)(aq) ions in the solution slowly react with H2O(l) in the reversible reaction represented below.
\(BF_4^-(aq)+H_{2}O(l)\rightleftharpoons BF_{3}OH^{-}(aq)+HF(aq)\)
The concentration of HF is monitored over time, as shown in the graph below.
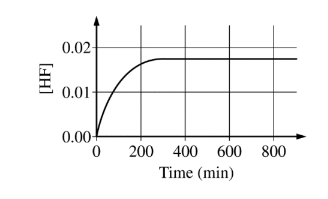
[HF] reaches a constant value of 0.0174 M when the reaction reaches equilibrium. For the forward reaction, the rate law is rate = kf \([BF_4^{-}\)]. The value of the rate constant kf was experimentally determined to be \(9.00\times 10^{-4}min^{-1}\).
(b) Calculate the rate of the forward reaction after 600. minutes. Include units with your answer. The rate law for the reverse reaction is rate = \(kr [BF_3OH^-]\)[HF].
(c) A student claims that the initial rate of the reverse reaction is equal to zero. Do you agree or disagree with this claim? Justify your answer in terms of the rate law for the reverse reaction.
(d) At equilibrium the forward and reverse reaction rates are equal. Calculate the value of the rate constant for the reverse reaction.
\(H_{3}BO_{3}(aq)+4HF(g)\rightarrow H_{3}O^{+}(aq)+BF_{4}^{-}(aq)+2H_{2}O(l)\)
Tetrafluoroboric acid is a strong acid with the formula \(HBF_4\). The acid can be prepared by reacting the weak acid \(H_3BO_3\) (molar mass 61.83 g/mol) with HF according to the equation above.
▶️Answer/Explanation
a(i) \(50.0gH_{3}BO_{3}\times \frac{1molH_{3}BO_{3}}{61.83g}\times \frac{1molBF_{4}^{-}}{1molH_{3}BO_{3}}\)
a(ii)\( 0.809 mol \times 4 = 3.24 mol\)
PV= nRT
\(V=\frac{nRT}{p}=\frac{(3.24mol)(0.08206Latm mol^{-1}K^{-1})(273K)}{1.00atm}\)
=72.6L
OR
\(0.809 mol \times 4 = 3.24 mol\)
\(\frac{22.4L}{1mol}\times 3.24mol=72.6 L\)
a(iii) As the reaction proceeds,\( H_3O^{+}\) is produced, so the pH
will decrease.
(b) \([BF_4^{-}] = 0.150 M – 0.0174 M = 0.133 M\)
\(rate = (9.00\times 10^{-4}min^{-1})(0.133M)=1.20\times 10^{-4}M min^{-1}\)
(c) Agree. The initial concentration of each product is zero, so the initial rate of the reverse reaction is zero.
(d ) 
Question
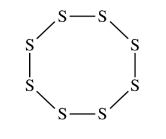
Elemental sulfur can exist as molecules with the formula \(S_8\) . The \(S_8\) molecule is represented by the incomplete Lewis diagram above.
(a) The diagram of \(S_8\) shows only bonding pairs of electrons. How many lone pairs of electrons does each S atom in the molecule have?
(b) Based on your answer to part (a), determine the expected value of the S–S–S bond angles in the S8 molecule.
(c) Write the electron configuration for the S atom in its ground state.
(d) The complete photoelectron spectrum for the element chlorine is represented below. Peak X in the spectrum corresponds to the binding energy of electrons in a certain orbital of chlorine atoms. The electrons in this orbital of chlorine have a binding energy of 273 MJ/mol, while the electrons in the same orbital of sulfur atoms have a binding energy of 239 MJ/mol.
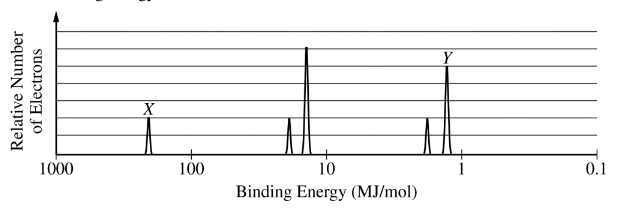
(i) Identify the orbital and explain the difference between the binding energies in terms of Coulombic forces.
(ii) Peak Y corresponds to the electrons in certain orbitals of chlorine atoms. On the spectrum shown, carefully draw the peak that would correspond to the electrons in the same orbitals of sulfur atoms.
\(3S_{8}+8OH^{-}\rightarrow 8S_{3}^{-}+4HOOH\)
In an experiment, a student studies the kinetics of the reaction represented above and obtains the data shown in the following table.
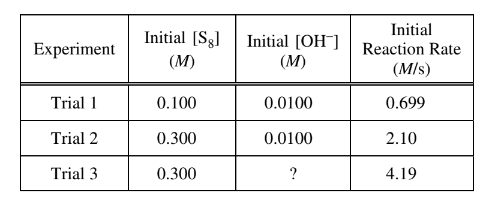
(e) Use the data in the table to do the following.
(i) Determine the order of the reaction with respect to \(S_8\) . Justify your answer.
(ii) Determine the value of\( [OH^−]\) that was used in trial 3, considering that the reaction is first order with respect to \(OH^−\). Justify your answer. The next day the student conducts trial 4 using the same concentrations of S 8 and \(OH^−\) as in trial 1, but the reaction occurs at a much slower rate than the reaction in trial 1. The student observes that the temperature in the lab is lower than it was the day before.
(f) Using particle-level reasoning, provide TWO explanations that help to account for the fact that the reaction rate is slower in trial 4.
▶️Answer/Explanation
(a) Two
(b)\(109.5^{\circ}\)
Acceptable range: \(104^{\circ}\leq angle \leq 110^{\circ}\).
(The experimentally determined angle is \(107^{\circ}8\)
(c) \(1s^{2} 2s^{2}2p^63s^2 3p^4\)
OR [Ne]\(3s^23p^4\)
(i) Peak X represents electrons in a 1s orbital. A Cl atom has one more proton in its nucleus than does a S atom; therefore, the electrons in Cl are more strongly attracted to the nucleus, and the binding energy of the 1s electrons in the Cl atom is greater than that of the 1s electrons in the S atom.
(ii) See example of a correct response (dashed peak) above.
(e)(i)
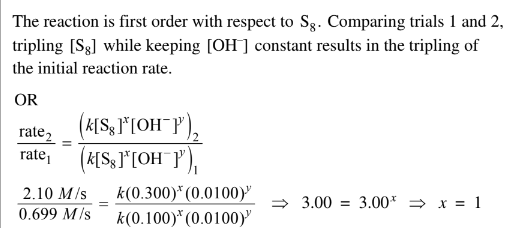
e(ii) Comparing trials 2 and 3, [S8] is kept constant and the initial reaction rate doubles. Since the reaction is first order with respect to\( OH^{-}\), the concentration of \(OH^{-}\) intrial 3 must be\( 2 \times 0.0100 M \)= 0.0200 M.
(f) The temperature was lower on the second day so the average kinetic energy of the reactant particles was lower. Therefore, there were fewer collisions between particles with sufficient
energy to react. Since the temperature was lower, the kinetic energy was lower and the average speed of the particles was lower. At the lower speeds, the reactant particles collided less frequently.
