Questions
Which of the following best explains why the combustion reactions represented in the table are exothermic?
(A) The number of bonds in the reactant molecules is greater than the number of bonds in the product molecules.
(B) The number of bonds in the reactant molecules is less than the number of bonds in the product molecules.
(C) The energy required to break the bonds in the reactants is greater than the energy released in forming the bonds in the products.
(D) The energy required to break the bonds in the reactants is less than the energy released in forming the bonds in the products.
▶️Answer/Explanation
Ans: D
Combustion reactions are exothermic because the energy released in forming the new bonds in the products (CO2 and H2O) is greater than the energy required to break the bonds in the reactants (hydrocarbons and O2).
Therefore, the correct answer is (D) The energy required to break the bonds in the reactants is less than the energy released in forming the bonds in the products.
Question
\(2 NO_2(g) → N_2O_4\) (g)
dark brown colorless
The dimerization of \(NO _2\)(g), an exothermic process, is represented by the equation above.
A mixture of \(NO _2\)(g) and \(N_2O_4\)(g) is at equilibrium in a rigid reaction vessel. If the temperature of the mixture is decreased, then
(A)\( [NO_2]\) will increase and the mixture will turn a darker brown
(B)\( [N_2O_4]\) will increase and the mixture will turn a lighter brown
(C) \( [N_2O_4]\) will decrease and the mixture will turn a lighter brown
(D) no change will be observed
▶️Answer/Explanation
Ans:B
The correct answer is (B) \([N_2O_4]\) will increase and the mixture will turn a lighter brown.
The dimerization of \(NO_2\) to form \(N_2O_4\) is an exothermic process, which means it releases heat. According to Le Chatelier’s principle, if an external stress is applied to a system at equilibrium, the system adjusts in such a way that the stress is partially offset.
So, if the temperature of the mixture is decreased, the system will try to counteract this change by producing more heat. It can do this by shifting the equilibrium to the right, towards the exothermic direction. This means more \(N_2O_4\) will be formed, and since \(N_2O_4\) is colorless, the mixture will turn a lighter brown. Therefore, option (B) is correct.
Question
\(2 NO_2(g) → N_2O_4\) (g)
dark brown colorless
The dimerization of NO2(g), an exothermic process, is represented by the equation above.
The forward reaction is thermodynamically favored at which of the following temperatures?
(A) All temperatures
(B) Low temperatures only
(C) High temperatures only
(D) No temperature
▶️Answer/Explanation
Ans:B
The dimerization of \(NO_2(g)\) to form \(N_2O_4(g)\) is an exothermic process, meaning it releases heat. According to Le Chatelier’s principle, for an exothermic reaction, increasing the temperature will shift the equilibrium position towards the reactants, favoring the reverse reaction. Conversely, decreasing the temperature will favor the forward reaction, leading to the formation of more \(N_2O_4(g)\).
Therefore, the forward reaction is thermodynamically favored at low temperatures. Thus, the correct answer is:
(B) Low temperatures only
Questions
\(2H_{2}O_{2}(l)\rightarrow 2H_{2}O(l)+O_{2(g)}\)
The exothermic process represented above is best classified as a
(A) physical change because a new phase appears in the products
(B) physical change because O2(g) that was dissolved comes out of solution
(C) chemical change because entropy increases as the process proceeds
(D) chemical change because covalent bonds are broken and new covalent bonds are formed
▶️Answer/Explanation
Ans: D
The given reaction involves the decomposition of hydrogen peroxide (\(H_{2}O_{2}\)) into water (\(H_{2}O\)) and oxygen gas (\(O_{2}\)).
Option (A): Physical changes typically involve changes in state or appearance without altering the chemical composition of the substance. In this reaction, while a phase change does occur (from liquid to gas for \(O_{2}\)), the chemical composition of the substances changes, so it’s not purely a physical change.
Option (B): This option suggests that the appearance of \(O_{2}(g)\) is due to it coming out of solution. However, the appearance of \(O_{2}(g)\) is due to its formation as a product of a chemical reaction rather than coming out of a solution. So, this option is not accurate.
Option (C): Entropy increasing doesn’t necessarily classify a reaction as chemical or physical. While entropy often increases in chemical reactions, it’s not a definitive indicator of a chemical change.
Option (D): Chemical changes involve the breaking and forming of chemical bonds, resulting in the formation of new substances with different chemical properties. In this reaction, covalent bonds in \(H_{2}O_{2}\) are broken to form \(H_{2}O\) and \(O_{2}\), indicating a chemical change.
So, the best classification for the given reaction is:
\[
\text{(D)} \quad \text{chemical change because covalent bonds are broken and new covalent bonds are formed}
\]
Questions

The first ionization energy of an element is the energy required to remove an electron from a gaseous atom of the element (i.e., \(X_{g}\rightarrow X^{+}_{g}+e^{-}\)”). The values of the first ionization energies for the third-row elements are shown in the graph above. On the basis of the information given, which of the following reactions is exothermic?
(A)\(Cl(_{g})+Mg^{+}(_{g})\rightarrow Cl^{+}(_{g})+Mg(_{g})\)
(B) \(Al(_{g})+Mg^{+}(_{g})\rightarrow Al^{+}(_{g})+Mg(_{g})\)
(C) \(P(_{g})+Mg^{+}(_{g})\rightarrow P^{+}(_{g})+Mg(_{g})\)
(D)\(S(_{g})+Mg^{+}(_{g})\rightarrow S^{+}(_{g})+Mg(_{g})\)
▶️Answer/Explanation
Ans: B
To determine which reaction is exothermic, we need to compare the first ionization energies of the elements involved in each reaction.
An exothermic reaction is one where energy is released, so the ionization of the atom on the reactant side should have a higher ionization energy than the ionization of the atom on the product side.
(A) \( \text{Cl(g)} + \text{Mg}^+(g) \rightarrow \text{Cl}^+(g) + \text{Mg(g)} \)
This reaction involves the ionization of Cl to \( \text{Cl}^+ \) (reactant side) and the reverse process of \( \text{Mg}^+ \) gaining an electron to form Mg (product side).
From the graph, the first ionization energy of Cl is higher than that of Mg, indicating that this reaction is endothermic (energy is absorbed).
(B) \( \text{Al(g)} + \text{Mg}^+(g) \rightarrow \text{Al}^+(g) + \text{Mg(g)} \)
This reaction involves the ionization of Al to \( \text{Al}^+ \) (reactant side) and the reverse process of \( \text{Mg}^+ \) gaining an electron to form Mg (product side).
From the graph, the first ionization energy of Al is lower than that of Mg, suggesting that this reaction is exothermic (energy is released).
(C) \( \text{P(g)} + \text{Mg}^+(g) \rightarrow \text{P}^+(g) + \text{Mg(g)} \)
This reaction involves the ionization of P to \( \text{P}^+ \) (reactant side) and the reverse process of \( \text{Mg}^+ \) gaining an electron to form Mg (product side).
From the graph, the first ionization energy of P is higher than that of Mg, indicating that this reaction is endothermic (energy is absorbed).
(D) \( \text{S(g)} + \text{Mg}^+(g) \rightarrow \text{S}^+(g) + \text{Mg(g)} \)
This reaction involves the ionization of S to \( \text{S}^+ \) (reactant side) and the reverse process of \( \text{Mg}^+ \) gaining an electron to form Mg (product side).
From the graph, the first ionization energy of S is higher than that of Mg, indicating that this reaction is endothermic (energy is absorbed).
Therefore, the correct answer is (B) \( \text{Al(g)} + \text{Mg}^+(g) \rightarrow \text{Al}^+(g) + \text{Mg(g)} \), as this is the only reaction that is exothermic based on the given first ionization energy values.
Question
\(HCl(aq)+NH_3(aq)→NH_4^+(aq)+Cl^−(aq)\)
The chemical reaction between HCl(aq) and \(NH_3\)(aq) is represented above. A student combines equimolar amounts of HCl(aq) and \(NH_3\)(aq), both solutions initially at 24°C, in a coffee-cup calorimeter. The student observes that the mixture reaches a temperature of 28°C . Based on the experimental results, which of the following can be concluded about the reaction?
A It is an endothermic process, because energy is released by the reaction and is gained by the reaction mixture.
B It is an endothermic process, because energy is absorbed by the reaction and is lost from the reaction mixture.
C It is an exothermic process, because energy is released by the reaction and is gained by the reaction mixture.
D It is an exothermic process, because energy is absorbed by the reaction and is lost from the reaction mixture.
▶️Answer/Explanation
Ans: C
In this case, the system can be defined as the reactants. As the reaction occurs, heat is released and is absorbed by the surroundings. As the mixture absorbs heat, the temperature reading increases. Therefore, the reaction is an exothermic process.
Question
When pellets of NaOH(s) are added to a flask of water, it is observed that the temperature of the water increases as the pellets dissolve. Which of the following claims about the observed dissolution of NaOH(s) in water is most accurate?
A It is an endothermic process because heat energy is absorbed by the water as the NaOH(s) dissolves in it.
B It is an endothermic process because heat energy is released by the water as the NaOH(s) dissolves in it.
C It is an exothermic process because heat energy is absorbed by the water as the NaOH(s) dissolves in it.
D It is an exothermic process because heat energy is released by the water as the NaOH(s) dissolves in it.
▶️Answer/Explanation
Ans:C
The dissolution of NaOH(s) is an exothermic process. The heat energy released as the NaOH(s) dissolves is absorbed by the water, resulting in an increase in the temperature of the water.
Question
An advertisement for a commercial hand warmer claims that the hand warmer works because a chemical reaction in the hand warmer draws out the body’s own natural heat, causing a warming effect. Which of the following states the accuracy of the claim in the advertisement and best provides a correct scientific justification of the claim?
A The advertisement’s claim is inaccurate because heat flowing from the hands to the warmer would only happen if the chemical reaction was endothermic, which would cause the hands to feel colder.
B The advertisement’s claim is inaccurate because heat flowing from the hands to the warmer would only happen if the chemical reaction was exothermic, which would cause the hands to feel colder.
C The advertisement’s claim is accurate because heat flowing from the hands to the warmer would only happen if the chemical reaction was endothermic, which would cause the hands to feel warmer.
D The advertisement’s claim is accurate because heat flowing from the hands to the warmer would only happen if the chemical reaction was exothermic, which would cause the hands to feel warmer.
▶️Answer/Explanation
Ans: A
The advertisement’s claim is inaccurate because if heat flowed from the hands to the warmer, the hands would get colder. The chemical reaction is exothermic, and the direction of heat flow is from the warmer to the hands, which causes the hands to feel warmer.
Question
\(2H_{2}O(l)\rightleftharpoons H_{3}O^{+}(aq)+OH^{-}(aq)\)
The autoionization of water is represented by the equation above. Values of \(pK_{w}\), at various temperatures are listed in the table below.
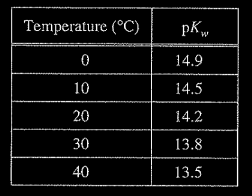
Based on the information above, which of the following statements is true?
(A) The dissociation of water is an exothermic process.
(B) The pH of pure water is 7.00 at any temperature.
(C) As the temperature increases, the pH of pure water increases.
(D) As the temperature increases, the pH of pure water decreases.
▶️Answer/Explanation
Ans:D
Question.
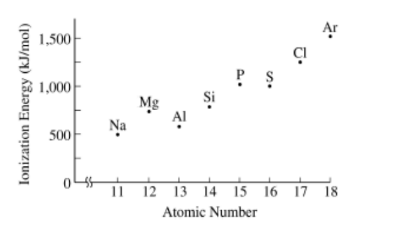
The first ionization energy of an element is the energy required to remove an electron from a gaseous atom of the element (i.e.,\( X(g) → X^{+}(g) + e ^-)\). The values of the first ionization energies for the third-row elements are shown in the graph above. On the basis of the information given, which of the following reactions is exothermic?
(A) \(Cl(g) + Mg+(g) → CI^{+}(g) + Mg(g)\)
▶️Answer/Explanation
Ans:B