Question
\(1s^2 2s^2 2p^6 3s^2 3p^6\)
How many unpaired electrons are in the atom represented by the electron configuration above?
B 1
C 2
D 3
▶️Answer/Explanation
Ans: A The 1s,2s,2p,3s, and 3p orbitals are all full, and all the electrons are paired.
Question
\(1s^2 2s^2 2p^6 3s^2 3p^6\)
How many unpaired electrons are in the atom represented by the electron configuration above?
B 1
C 2
D 3
▶️Answer/Explanation
Ans: A The 1s,2s,2p,3s, and 3p orbitals are all full, and all the electrons are paired.
Question
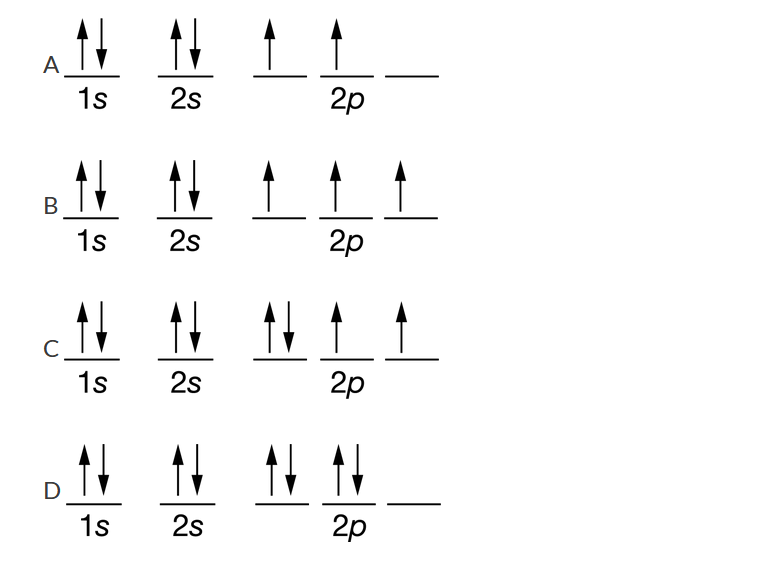
Which of the following represents the electron configuration of an oxygen atom in the ground state?
▶️Answer/Explanation
Ans:C This configuration contains eight electrons. According to Hund’s rule, each orbital in the last subshell to have electrons placed in it (in this case the 2p subshell) has one electron placed in it before pairing of electrons in orbitals occurs.
Question
Which of the following is the correct electron configuration for a ground-state atom of magnesium (atomic number 12) ?
A \(1s^22s^22p^8\)
B \(1s^22s^23s^23p^6\)
C \(1s^22s^22p^63s^2\)
D \(1s^22s^23s^43p^4\)
▶️Answer/Explanation
Ans: C This electron configuration has the proper sequence of orbitals filled with the correct number of electrons.
Question
The charge on an electron was determined in the __________.
A) Rutherford gold foil experiment
B) Dalton atomic theory
C) cathode ray tube, by J. J. Thompson
D) atomic theory of matter
E) Millikan oil drop experiment
▶️Answer/Explanation
Ans: E
Question
All of the orbitals in a given electron shell (energy level) have the same value of the __________ quantum
number.
A) spin B) principal C) azimuthal D) magnetic E) psi
▶️Answer/Explanation
Ans: B
