Question
\(2NO_{2}(g)+F_{2}(g)\rightarrow 2NO_{2}F(g)\)
The rate law for the reaction represented by the equation above is rate = k \([NO_{2}][F_{2}]\). Which of the following could be the first elementary step of a two-step mechanism for the reaction if the first step is slow and the second step is fast?
(A) \(F_{2}(g)\rightarrow 2F(g)\)
(B) \(NO_{2}(g)+F_{2}(g)\rightarrow NO_{2}F(g) + F(g)\)
(C) \(NO_{2}(g)+F_{2}(g)\rightarrow NO_{2}F(g)\)
(D) \(2NO_{2}(g)+F_{2}(g)\rightarrow 2NO_{2}F(g)\)
▶️Answer/Explanation
Ans:C
Question
\(2NO_{2}(g)+F_{2}(g)\rightarrow 2NO_{2}F(g)\)
The rate law for the reaction represented by the equation above is rate = k \([NO_{2}][F_{2}]\). Which of the following could be the first elementary step of a two-step mechanism for the reaction if the first step is slow and the second step is fast?
(A) \(F_{2}(g)\rightarrow 2F(g)\)
(B) \(NO_{2}(g)+F_{2}(g)\rightarrow NO_{2}F(g) + F(g)\)
(C) \(NO_{2}(g)+F_{2}(g)\rightarrow NO_{2}F(g)\)
(D) \(2NO_{2}(g)+F_{2}(g)\rightarrow 2NO_{2}F(g)\)
▶️Answer/Explanation
Ans:C
Question.
\(0_{3}(g) + O(g) → 2O_{2}\)(g)
The decomposition of \(O_{3}\)(g) in the upper atmosphere is represented by the equation above. The potential energy diagram for the decomposition of \(O_{3}\)(g) in the presence and absence of \(NO_{3}\)(g) is given below.
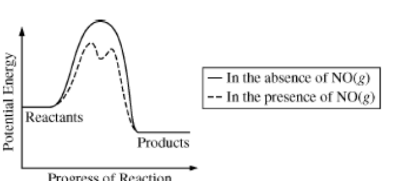
Which of the following mechanisms for the catalyzed reaction is consistent with the equation and diagram above?
(A) \(2O_{3}(g) +2 NO(g) → 4 O_{2}(g) +N_{2}(g)\) slow
(B) \(O_{3}(g) +NO(g) →→ NO_{2}(g) + O_{2}(g)\) slow
\(NO_{2}(g) + O(g) → NO(g) + O_{2}(g)\) fast
(C) \(NO_{2}(g) +O_{3}(g) → NO(g) +2O_{2}\)(g) slow
\(NO(g) + O(g) → NO_{2}\)(g) fast
(D)\( NO_{2}(g) + O(g) → NO_{3}\)(g) slow
\(NO_{3}(g) +O_{3}(g) → NO_{2}(g) +2 O_{2}\)(g) fast
▶️Answer/Explanation
Ans:C
Question
(\(H_{2}(g) + Cl_{2}(g) \rightleftharpoons 2HCl\)(g) \(K_{p} = 2× 10^{30}\) at 298 K
HCI(g) can be synthesized from\( H_{2}(g)\) and \(Cl_{2}(g)\) as represented above. A student studying the kinetics of the reaction proposes the following mechanism.
Step 1: \(Cl_{2}(g) → 2 Cl(g)\) (slow) \(\Delta H^{\circ} = 242 kJ/mol_{rxn}\)
Step 2:\( H_2(g) + Cl(g) → HCl(g) + H(g)\) (fast) \(\Delta H^{\circ} = 4 kJ/mol_{rxn}\).
Step 3: H(g) + Cl(g) → HCl(g) (fast) \(\Delta H^{\circ} = -432 kJ/mol_{rxn}\).
Which of the following statements identifies the greatest single reason that the value of \(K_{p}\) for the overall reaction at 298 K has such a large magnitude?
(A) The activation energy for step 1 of the mechanism is large and positive.
(B) The activation energy for step 2 of the mechanism is small and positive.
(C) The value of\( \Delta S^{\circ}\) for the overall reaction is small and positive.
(D) The value of \(\Delta H^{\circ}\) for the overall reaction is large and negative.
▶️Answer/Explanation
Ans:A
Question
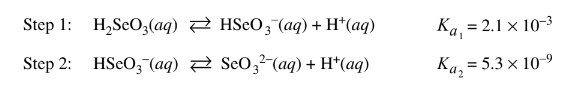
The step-wise dissociation of selenous acid,\( H_2SeO_3\)(aq), is represented by the equations above. Which of the following best helps explain why the value of \(K_{a2}\) is so much smaller than the value of \(K_{a1}\) ?
(A) The Se atom in \(H_{2}SeO_{3}\)(aq) is in a higher oxidation state than the Se atom in \(HSeO_{3}^{−}\)(aq).
(B) Water is more attracted to \(HSeO_{3}^{−}~(aq)\) ions than to \(SeO_{3}^{2−}\) ions, which drives the step 2 equilibrium toward the reactant.
(C) The\( HSeO_{3}^{−}\)(aq) ions produced in step 1 are asymmetrical, but the \(SeO_{3} ^{2− }\)ions produced in step 2 are symmetrical.
(D) Removing the first \(H^{+}\) from \(H_{2}SeO_{3}\)(aq) requires less energy than removing the second \(H^{+}\), because the second\( H^{+}\) is removed from a negatively charged species.\(Li_{3}N{s}+2H_{2}(g)\rightleftharpoons LiNH_{2}(s)+2LiH(s) \Delta H \)=\(-192kj/mol_{rxn}\) Because pure \(H_2\) is a hazardous substance, safer and more cost effective techniques to store it as a solid for shipping purposes have been developed. One such method is the reaction represented above, which occurs at 200°C.
▶️Answer/Explanation
Ans:D
Question
The mechanism for formation of the product X is:
A + B \(\rightarrow \) C + D (slow)
B + D \(\rightarrow \) X (fast)
The intermediate reactant in the reaction is __________.
A) A B) B C) C D) D E) X
▶️Answer/Explanation
Ans: D
