Questions
Which of the following best helps to explain why Na(s) is more reactive with water than Mg(s) is?
(A) Na(s) is softer than Mg(s).
(B) The atomic mass of Na is less than that of Mg.
(C) The \(Na^{+}\) ion has weaker Coulombic attraction to an anion than the\( Mg^{ 2+}\) ion has.
(D) The first ionization energy of Na is less than that of Mg.
▶️Answer/Explanation
Ans: C
The reactivity of alkali metals like sodium (Na) and alkaline earth metals like magnesium (Mg) with water can be explained by their respective properties.
(A) Na(s) is softer than Mg(s).
This statement is not directly relevant to their reactivity with water. Softness refers to the ease with which a material can be deformed or shaped, which doesn’t necessarily correlate with reactivity with water.
(B) The atomic mass of Na is less than that of Mg.
While atomic mass can influence reactivity, it’s not the primary factor in this case. Reactivity with water is more influenced by other factors like ionization energy and the strength of the metallic bond.
(C) The \(Na^+\) ion has weaker Coulombic attraction to an anion than the \(Mg^{2+}\) ion has.
This statement accurately explains the difference in reactivity. When alkali metals react with water, they lose an electron to form a positive ion (cation). The strength of attraction between the resulting cation
and water molecules influences the reactivity. Since sodium forms \(Na^+\) ions, which have weaker Coulombic attraction to anions compared to \(Mg^{2+}\) ions, sodium reacts more vigorously with water.
(D) The first ionization energy of Na is less than that of Mg.
This statement is also relevant to their reactivity. The first ionization energy refers to the energy required to remove the outermost electron from an atom. Sodium has a lower first ionization energy compared to
magnesium, meaning it is easier to remove an electron from a sodium atom. This makes sodium more reactive with water as it readily loses electrons to form \(Na^+\) ions.
So, the most relevant explanation for why Na(s) is more reactive with water than Mg(s) is:
(C) The \(Na^+\) ion has weaker Coulombic attraction to an anion than the \(Mg^{2+}\) ion has.
Question
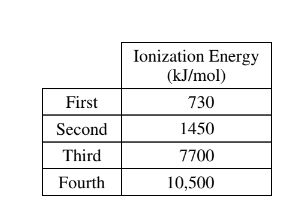
The ionization energies of an unknown element, X, are listed in the table above. Which of the following is the most likely empirical formula of a compound formed from element X and phosphorus, P ?
(A) \(XP\)
(B) \(X_{3}P\)
(C) \(X_{3}P_{2}\)
(D) \(X_{3}P_{4}\)
▶️Answer/Explanation
Ans:C
To determine the most likely empirical formula of a compound formed between element X and phosphorus (P), we need to consider the ionization energies and the general rules for chemical bonding.
The ionization energies provided in the table show a significant jump between the third and fourth ionization energies. This pattern is typical of elements in the third period of the periodic table, where the first three ionization energies correspond to the removal of electrons from the outer valence shell, and the fourth ionization energy corresponds to the removal of an electron from the next inner shell.
Based on this information, we can infer that element X is likely a Group 13 element (like boron, aluminum, gallium, indium, or thallium) with three valence electrons.
Group 13 elements typically form compounds with a +3 oxidation state, meaning they would share three electrons in chemical bonds.
Phosphorus (P) is a Group 15 element with five valence electrons, and it commonly forms compounds with a -3 oxidation state, meaning it would share three electrons in chemical bonds.
To balance the charges and achieve a stable octet configuration, the most likely empirical formula would involve three atoms of element X donating three electrons each to share with two atoms of phosphorus (P), resulting in the formula X₃P₂.
Therefore, the most likely empirical formula of a compound formed from element X and phosphorus is (C) (X_{3}P_{2}\).
Questions
The compound \(CCl_{4}\) is nonflammable and was once commonly used in fire extinguishers. On the basis of the periodic properties, which of the following compounds can most likely be used as a fire-resistant chemical?
(A) \(BCl_{3}\)
(B) \(CH_{4}\)
(C) \(CBr_{4}\)
(D) \(PbCl_{2}\)
▶️Answer/Explanation
Ans: C
To determine which compound can most likely be used as a fire-resistant chemical, we need to consider the properties of the elements involved and their tendency to form compounds with high thermal stability and low reactivity.
A) \(BCl_{3}\) (Boron trichloride)
Boron trichloride is a reactive and toxic compound that readily hydrolyzes in the presence of water vapor, forming hydrogen chloride (HCl) and boric acid. It is not a suitable fire-resistant chemical due to its reactivity.
B) \(CH_{4}\) (Methane)
Methane is a flammable gas, and it is the main component of natural gas. It is not a fire-resistant chemical.
C) \(CBr_{4}\)(Carbon tetrabromide)
Carbon tetrabromide is a nonflammable and thermally stable compound, similar to carbon tetrachloride (CCl4). The presence of the highly electronegative bromine atoms can help reduce the compound’s reactivity and flammability. Carbon tetrabromide is a reasonable choice as a fire-resistant chemical.
D) \(PbCl_{2}\) (Lead(II) chloride)
Lead(II) chloride is an ionic compound that is thermally stable and nonflammable. However, lead compounds are generally toxic and have negative environmental impacts, making them less desirable for use as fire-resistant chemicals.
Based on the periodic properties and the stability of carbon-halogen compounds, the most likely choice for a fire-resistant chemical is (C) CBr4 (Carbon tetrabromide).
Like carbon tetrachloride, carbon tetrabromide is nonflammable and has high thermal stability due to the strong carbon-halogen bonds and the high electronegativity of the halogen atoms. The bromine atoms in CBr4 contribute to its nonflammable nature and make it a suitable fire-resistant chemical.
Questions
Which of the following has the bonds arranged in order of decreasing polarity?
(A) H-F > N-F > F-F
(B) H-I > H-Br > H-F
(C) O-N > O-S > O-Te
(D) Sb-I > Sb-Te > Sb-Cl
▶️Answer/Explanation
Ans: A
To determine the order of decreasing polarity among the given bond pairs, we need to consider the electronegativity difference between the atoms forming the bonds. Generally, a greater electronegativity difference between atoms results in a more polar bond.
(A) H-F > N-F > F-F:
The H-F bond has a large electronegativity difference between hydrogen (2.20) and fluorine (3.98), making it highly polar.
The N-F bond also has a significant electronegativity difference, but it is generally less polar than the H-F bond.
The F-F bond has the same element on both sides, so it is nonpolar.
(B) H-I > H-Br > H-F:
The H-I bond has a smaller electronegativity difference compared to the H-F bond in option (A), so it is less polar.
The H-Br bond has a smaller electronegativity difference compared to the H-I bond, so it is even less polar.
The H-F bond is the most polar among the three.
(C) O-N > O-S > O-Te:
The electronegativity difference decreases going from O-N to O-Te, so the polarity of the bonds decreases accordingly.
(D) Sb-I > Sb-Te > Sb-Cl:
Similar to option (C), the electronegativity difference decreases going from Sb-I to Sb-Cl.
Therefore, the correct answer is option (A) H-F > N-F > F-F, which presents the bonds arranged in order of decreasing polarity.
Question
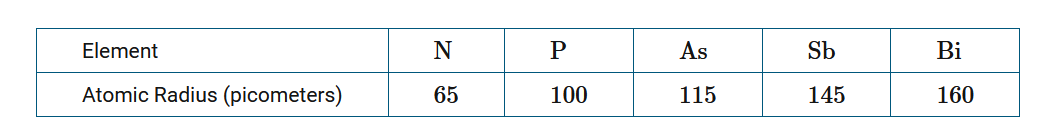
The atomic radii of the elements in the nitrogen group in the periodic table are given in the table above. Which of the following best helps explain the trend of increasing atomic radius from N to Bi ?
▶️Answer/Explanation
Ans:C Going down the group, the net attraction of the valence electrons for the nucleus decreases; thus the valence electrons maintain a greater average distance from the nucleus.