Question

A student is given the task of determining the I content of tablets that contain KI and an inert, water-soluble sugar as a filler. A tablet is dissolved in 50.0 mL of distilled water, and an excess of 0.20 M\( Pb(NO_3)_2\)(aq) is added to the solution. A yellow precipitate forms, which is then filtered, washed, and dried. The data from the experiment are shown in the table above.
(a) For the chemical reaction that occurs when the precipitate forms, (i) write a balanced, net-ionic equation for the reaction, and
(ii) explain why the reaction is best represented by a net-ionic equation.
(b) Explain the purpose of drying and weighing the filter paper with the precipitate three times.
(c) In the filtrate solution, is [K] greater than, less than, or equal to Justify \([NO_{3}^{-}]\)? your answer.
(d) Calculate the number of moles of precipitate that is produced in the experiment.
(e) Calculate the mass percent of I in the tablet.
(f) In another trial, the student dissolves a tablet in 55.0 mL of water instead of 50.0 mL of water. Predict whether the experimentally determined mass percent of I will be greater than, less than, or equal to the amount calculated in part
(e). Justify your answer.
g) A student in another lab also wants to determine the I content of a KI tablet but does not have access to Pb\((NO_3)_2\). However, the student does have access to 0.20 M\( AgNO_3\), which reacts with I ̄(aq) to produce AgI(s). The value of Kp for Agl is 8.5 × 10−17.
(i) Will the substitution of \(AgNO_3\) for \(Pb(NO_3)_2\) result in the precipitation of the I- ion from solution? Justify your answer.
(ii) The student only has access to one KI tablet and a balance that can measure to the nearest 0.01 g. Will the student be able to determine the mass of Agl produced to three significant figures? Justify your answer.
▶️Answer/Explanation
a.(i) \(Pb^{2+} + 2I^{-} → Pbl_{2}\)
a.(ii) The net-ionic equation shows the formation of the\( Pbl_{2}\)(s) from \(Pb^{2+}\)(aq) and \(I^{+}\)(aq) ions, omitting the non-reacting species (spectator ions), \(K^{+}\)(aq) and \(NO_{3} ̄\)(aq).
(b) The filter paper and precipitate must be dried several times (to a constant mass) to ensure that all the water has been driven off.
(c) [\(K^{+}\)] is less than \([NO_{3}\)] because the source of the\( NO_{3}^{-}\), the 0.20 M Pb(NO_{3})_{2}(aq), was added in excess.
(d)1.698 g 1.462 g=0.236 g Pbl2(s)
\(0.236g Pbl_{2}\frac{1molPbl_{2}}{461.0gPbl_{2}}=5.12\times 10^{-4} mol Pbl_{2}\)
(e) \(5.12 × 10^{-4}mol Pbl_{2} ×\frac{2 mol I^{-}}{1 mol Pbl_{2}}\)= 1.02 × 10^{-3} mol I^{-1}\)
\(1.02 x 10^{3}mol I ̄ ×\frac{126.91 g I^{-}}{1 mol I^{-}}=0.130 g I^{-}\) in one tablet
\(\frac{0.130 g I^{-}} {0.425 g KI tablet}\)= 0.306 30.6% per KI tablet
(f) The mass percent of\( I^{-}\) will be the same. \(Pb^{2+}\)(aq) was added in excess, ensuring that essentially no I remained in solution. The additional water is removed by filtration and drying, leaving the same mass of dried precipitate.
g (i) Yes. Addition of an excess of 0.20 M \(AgNO_{3}\)(aq) will precipitate all of the I- ion present in the solution because Agl is insoluble, as evidenced by its low value of \(K_{sp}\)
g.(ii) No. If masses can be measured to ±0.01 g, then the mass of the dry Agl(s) precipitate (which is less than 1 g) will be known to only two significant figures.
Question
2 NO(g) + O2(g) → 2 NO2(g)
A student investigates the reactions of nitrogen oxides. One of the reactions in the investigation requires an equimolar mixture of NO(g) and NO2(g), which the student produces by using the reaction represented above.
(a) The particle-level representation of the equimolar mixture of NO(g) and NO2(g) in the flask at the completion of the reaction between NO(g) and O2(g) is shown below in the box on the right. In the box below on the left, draw the particle-level representation of the reactant mixture of NO(g) and O2(g) that would yield the product mixture shown in the box on the right. In your drawing, represent oxygen atoms and nitrogen atoms as indicated below.
The student reads in a reference text that NO(g) and NO2(g) will react as represented by the equation below.
Thermodynamic data for the reaction are given in the table below the equation.
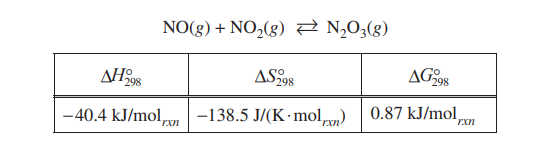
(b) The student begins with an equimolar mixture of NO(g) and NO2(g) in a rigid reaction vessel and the mixture reaches equilibrium at 298 K.
(i) Calculate the value of the equilibrium constant, K, for the reaction at 298 K.
(ii) If both PNO and PNO2 in the vessel are initially 1.0 atm, will PN2O3 at equilibrium be equal to 1.0 atm? Justify your answer.
(c) The student hypothesizes that increasing the temperature will increase the amount of N2O3(g) in the equilibrium mixture. Indicate whether you agree or disagree with the hypothesis. Justify your answer.
N2O3(g) reacts with water to form nitrous acid, HNO2 (aq), a compound involved in the production of acid rain.
The reaction is represented below.
N2O3(g) + H2O(l) → 2 HNO2(aq)
(d) The skeletal structure of the HNO2 molecule is shown in the box below.
(i) Complete the Lewis electron-dot diagram of the HNO2 molecule in the box below, including any lone pairs of electrons.

(ii) Based on your completed diagram above, identify the hybridization of the nitrogen atom in the HNO2 molecule. To produce an aqueous solution of HNO2 , the student bubbles N2O3(g) into distilled water. Assume that the reaction goes to completion and that HNO2 is the only species produced. To determine the concentration of HNO2(aq) in the resulting solution, the student titrates a 100. mL sample of the solution with 0.100 M KOH(aq).
The neutralization reaction is represented below.
HNO2(aq) + OH−(aq) → NO2−(aq) + H2O(l)
The following titration curve shows the change in pH of the solution during the titration.
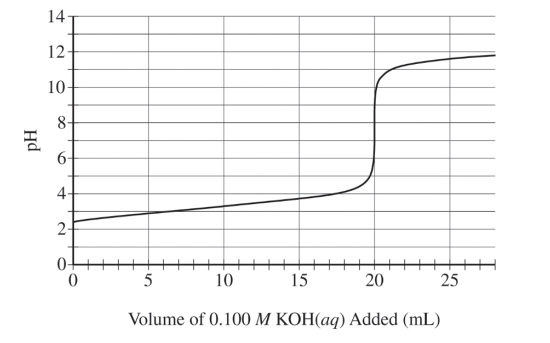
(e) Use the titration curve and the information above to
(i) determine the initial concentration of the HNO2(aq) solution
(ii) estimate the value of pKa for HNO2(aq)
(f) During the titration, after a volume of 15 mL of 0.100 M KOH(aq) has been added, which species, HNO2(aq) or NO2−(aq), is present at a higher concentration in the solution? Justify your answer.
▶️Answer/Explanation
Ans:
(a)
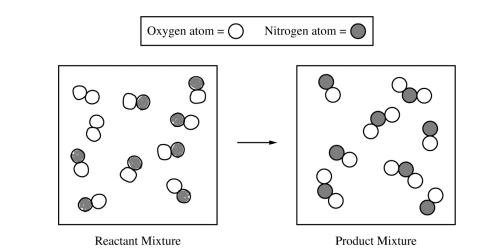
| See sample student response above. (8 molecules of NO and 2 molecules of O2) |
(b) (i)
ΔG0 = – RT In K \(K = e^{-\frac{870 J/mol}{(8.314 J mol^{-1}K^{-1})(298 K)}}\) |
(ii)
No, the pressure will not equal 1.0 atm. PN2O3 would only equal 1.0 atm if the reaction goes to completion. |
(c)
Disagree. Because the reaction is exothermic, increasing the temperature of the reaction will favor the formation of the reactants (according to Le Chatelier’s principle). |
(d)

(i)
| See sample response above. (Line segments can be used to represent electron pairs.) |
(ii)
| sp2 |
(e) (i)
20. mL \(KOH\times \frac{0.100 mol KOH}{1000 mL KOH}=0.0020 mol\) KOH added \(\frac{0.0020 mol HNO_{2}}{0.100 L}=0.020 M HNO_{2}\) |
(ii)
| The value of pKa is about 3.4. |
(f)
| NO2–(aq) The titration is past the half-equivalence point; therefore, there will be more conjugate base present than acid. |
