Questions

Two molecules of the amino acid glycine join through the formation of a peptide bond, as shown above. The thermodynamic data for the reaction are listed in the following table.

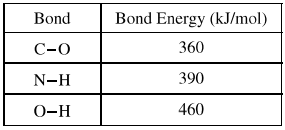
Based on the bond energies listed in the table above, which of the following is closest to the bond energy of the C-N bond?
(A) 200 kJ/mol
(B) 300 kJ/mol
(C) 400 kJ/mol
(D) 500 kJ/mol
▶️Answer/Explanation
Ans: B
The bond energies provided are:
\[ C-O: 360 \, \text{kJ/mol} \]
\[ N-H: 390 \, \text{kJ/mol} \]
\[ O-H: 460 \, \text{kJ/mol} \]
We can observe that the bond energy increases as the electronegativity difference between the bonded atoms increases. This trend is known as the “bond energy-electronegativity difference relationship.”
The electronegativity values (on the Pauling scale) for the elements involved are:
\[ \text{Carbon (C): } 2.5 \]
\[ \text{Nitrogen (N): } 3.0 \]
\[ \text{Oxygen (O): } 3.5 \]
\[ \text{Hydrogen (H): } 2.1 \]
The electronegativity difference between the bonded atoms is:
\[ C-O: 3.5 – 2.5 = 1.0 \]
\[ N-H: 3.0 – 2.1 = 0.9 \]
\[ O-H: 3.5 – 2.1 = 1.4 \]
We can see that the O-H bond has the highest electronegativity difference, followed by C-O and then N-H.
Since the C-N bond involves a smaller electronegativity difference than C-O and N-H, we can expect the C-N bond energy to be lower than the given values of \( 360 \, \text{kJ/mol} \) and \( 390 \, \text{kJ/mol} \).
Therefore, the option that is closest to the bond energy of the C-N bond is \( (B) \, 300 \, \text{kJ/mol} \).
Note: This answer is based on the general trend that bonds between atoms with smaller electronegativity differences tend to have lower bond energies compared to bonds between atoms with larger electronegativity differences.
Question
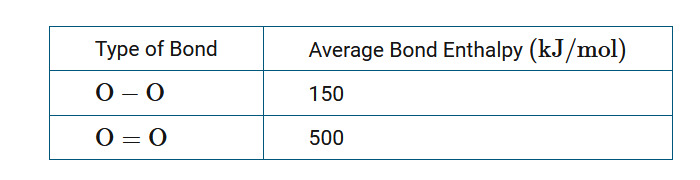
\(2O_3(g)→3O_2(g)\) \(\Delta H^{0}_{rxn}=-300kJ/mol_{rxn}\)
The conversion of ozone to diatomic oxygen is represented by the equation above. Based on the data in the table above, what is the approximate average bond enthalpy for the oxygen-to-oxygen bonds in ozone?
A 0kJ/mol
B 150kJ/mol
C 300kJ/mol
D 500kJ/mol
▶️Answer/Explanation
Ans:C
The oxygen-oxygen bond energy in ozone can be calculated by representing that energy as x . In two ozone molecules there are four oxygen-oxygen bonds that are broken in the reaction, and three O=O double bonds that are formed in the reaction. The sum of the energies of the bonds broken subtracted from the sum of the energies of the bonds formed is a good approximation of the enthalpy change for the reaction; thus, the equation 4x+3(−500)=−300 can be solved for x, which is 300kJ/mol.
Question
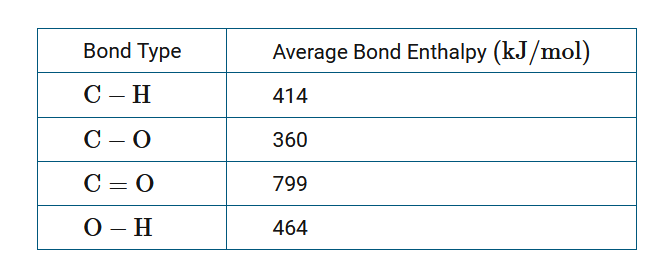
The combustion of methane proceeds according to the chemical equation \(CH_4+2 O_2→CO_2+2 H_2O\). The table above provides the average bond enthalpy for selected bonds. The calculated enthalpy change for the reaction, Δ\(H_{rxn}\), is −802 kJ/mol. Which of the following provides the mathematical procedure to estimate the bond enthalpy per mole of \(O_2\)?
A \(\frac{1}{2}[−802 +2(799)+4(464)−4(414)]kJ/mol\)
B \(2[−802+799+2(464)−2(414)]kJ/mol\)
C \(\frac{1}{2}[−802−2(360)+4(464)+4(414)]kJ/mol\)
D \(2[−802−360+2(464)+414]kJ/mol\)
▶️Answer/Explanation
Ans:A
To calculate the enthalpy change for a chemical reaction using average bond enthalpies (BE ), the general mathematical equation used is Δ\(H_{rxn}\)=Σ (number of moles of bonds broken)(\(BE_{type of bond broken}\))−Σ(number of moles of bonds formed)(\(BE_{type of bond formed}\)). In the reactants there are four moles of C−H bonds in one mole of \(CH_4\) and two moles of O=O bonds in two moles of \(O_2\). In the products there are two moles of C=O bonds in one mole of \(CO_2\) and four moles of O−H bonds in two moles of \(H_2O\). If x is the molar bond enthalpy of O2, and the enthalpy change of the reaction is −802kJ/mol, then −802=4(414)+2x−(2(799)+4(464)). Solving this equation for x yields the expression \(\frac{1}{2}[−802+2(799)+4(464)−4(414)]kJ/mol\).
Question
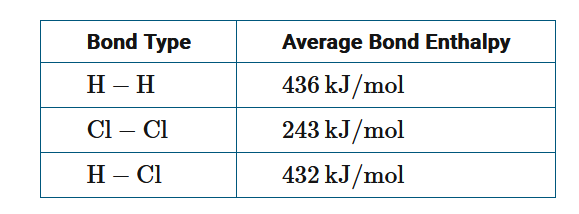
The formation of hydrogen chloride gas is represented by the chemical equation \(H_2(g)+Cl_2(g)→2HCl(g)\) . Based on the bond enthalpy data in the table above, what is the approximate enthalpy change for the reaction?
A −247\(kJ/mol_{rxn}\)
B −185\(kJ/mol_{rxn}\)
C +185\(kJ/mol_{rxn}\)
D +247\(kJ/mol_{rxn}\)
▶️Answer/Explanation
Ans:B
The use of average bond enthalpies along with the knowledge that 1 mole of H−H bonds and 1 mole of Cl−Cl bonds are broken (positive enthalpy value) while 2 moles of H−Cl bonds are formed (negative enthalpy value), gives Δ\(H_{rxn}=(436+243)−(2×432)=−185kJ/mol_{rxn}\).
Question
Which of the following arranges the molecules \(N_{2}\) , \(O_{2}\) , and \(F_{2}\) in order of their bond enthalpies, from least to greatest?
(A) \(F_{2}\) < \(O_{2}\) < \(N_{2}\)
(B) \( O_{2}\) < \(N_{2}\) <\( F_{2}\)
(C) \( N_{2}\) < \(O_{2}\) < \(F_{2}\)
(D) \(N_{2}\) <\( F_{2}\) < \(O_{2}\)
▶️Answer/Explanation
Ans:A
Question
\(CH_{3}OH(g) \rightarrow CO(g) + 2 H_{2}\)(g) \( \Delta H^{\circ}=+91KJ/mol_{rxn}\)
The reaction represented above goes essentially to completion. The reaction takes place in a rigid, insulated vessel that is initially at 600 K.
Which of the following statements about the bonds in the reactants and products is most accurate?
(A) The sum of the bond enthalpies of the bonds in the reactant is greater than the sum of the bond enthalpies of the bonds in the products.
(B) The sum of the bond enthalpies of the bonds in the reactant is less than the sum of the bond enthalpies of the bonds in the products.
(C) The length of the bond between carbon and oxygen in \(CH_{3}\)OH is shorter than the length of the bond between carbon and oxygen in CO.
(D) All of the bonds in the reactant and products are polar.
▶️Answer/Explanation
Ans:A