Questions
Which of the following modifications will increase the rate of the reaction the most?
(A) Using 2.0 M \(CH_{3}COOH\)(aq) instead of 2.0 M HCl(aq)
(B) Cooling the HCl(aq) to a lower temperature than it was in the original experiment
(C) Reducing the volume of the reaction vessel
(D) Using eggshells that are more finely powdered than those used in the original experiment
▶️Answer/Explanation
Ans: C
Since reaction is exothermic, so lowering temperature will increase rate of reaction Hence, cooling the HCl (aq.) to a lower temperture than it was in original experiment will increase the rate of reaction most \(\rightarrow\) option (B)
Question
\(N_2O(g) + CO(g) \)→ \(N_2(g) + CO_2(g)\)
The rate of the reaction represented above increases significantly in the presence of Pd(s). Which of the following best explains this observation?
(A) Pd increases the activation energy of the reaction.
(B) Pd absorbs the heat produced in the reaction.
(C) One of the reactants binds on the surface of Pd, which introduces an alternative reaction pathway with a lower activation energy.
(D) One of the products binds on the surface of Pd, which increases the reaction rate by decreasing the concentration of products in the mixture.
▶️Answer/Explanation
Ans:C
The significant increase in the rate of the reaction in the presence of Pd(s) suggests that Pd acts as a catalyst. A catalyst provides an alternative reaction pathway with lower activation energy, allowing the reaction to occur more readily without being consumed in the process.
(A) Pd increases the activation energy of the reaction.
This statement contradicts the concept of catalysis. Catalysts typically lower the activation energy required for a reaction, making it easier for the reaction to proceed.
(B) Pd absorbs the heat produced in the reaction.
This statement does not explain the observed increase in the rate of the reaction. Heat absorption or removal may affect reaction equilibrium or kinetics, but it does not typically serve as the primary mechanism for catalysis.
(C) One of the reactants binds on the surface of Pd, which introduces an alternative reaction pathway with a lower activation energy.
This statement aligns with the concept of catalysis. Catalysts often provide an alternative reaction pathway by facilitating the adsorption of reactant molecules onto their surface, allowing the reaction to proceed through a lower activation energy pathway.
(D) One of the products binds on the surface of Pd, which increases the reaction rate by decreasing the concentration of products in the mixture.
While product inhibition is a phenomenon observed in some reactions, it does not explain the observed increase in the rate of the reaction in the presence of Pd(s).
Therefore, the most suitable explanation for the observed increase in the reaction rate is: (C) One of the reactants binds on the surface of Pd, which introduces an alternative reaction pathway with a lower activation energy.
Question
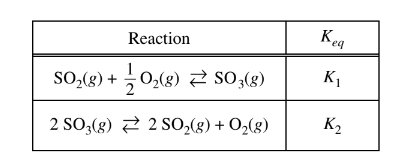
Which of the following shows the relationship between \(K_1\) and\( K_2\) in the reactions represented above?
(A) \(K_{2}\)= \((K_{1})^{2}\)
(B)\( K_{2}\)=\(\sqrt{K_{1}}\)
(C) \( K_{2}\)=\(\frac{1}{(K_{1})^{2}}\)
(D) \(K_{2}\)=\(\frac{1}{K_{1}}\)
▶️Answer/Explanation
Ans:C
To establish the relationship between \(K_1\) and \(K_2\) for the given reactions, we can use the principle of the equilibrium constant (\(K_{eq}\)).
For the first reaction:
\[\text{SO}_2(g) + \frac{1}{2} \text{O}_2(g) \rightleftarrows \text{SO}_3(g) \quad \text{(Equation 1)}\]
And for the second reaction:
\[2 \text{SO}_3(g) \rightleftarrows 2 \text{SO}_2(g) + \text{O}_2(g) \quad \text{(Equation 2)}\]
Given that \(K_1\) and \(K_2\) represent the equilibrium constants for these reactions, we can determine the relationship between them by considering the relationship between the equilibrium constants for the forward and reverse reactions.
For Equation 2, the equilibrium constant (\(K_2\)) is related to the equilibrium constant of the reverse reaction (Equation 1), which is \(K_{1}\).
Since Equation 2 is the reverse of Equation 1 and multiplied by 2, we have:
\( K_{2}\)=\(\frac{1}{(K_{1})^{2}}\)
Question
\[
\mathrm{Li}_3 \mathrm{~N}(s)+2 \mathrm{H}_2(g) \rightleftarrows \mathrm{LiNH}_2(s)+2 \mathrm{LiH}(s) \quad \Delta H^{\circ}=-192 \mathrm{~kJ} / \mathrm{mol}_{r x n}
\]
Because pure \(\mathrm{H}_2\) is a hazardous substance, safer and more cost effective techniques to store it as a solid for shipping purposes have been developed. One such method is the reaction represented above, which occurs at \(200^{\circ} \mathrm{C}\).
Which of the following is the most likely reason that the reaction occurs at a significant rate only if the temperature of the reaction mixture is greater than 200°C?
(A) The reaction is exothermic.
(B) ΔS° for the reaction is negative.
(C) The reaction has a high activation energy.
(D) ΔG° < 0 whenT < 200°C.
▶️Answer/Explanation
Ans:C
The temperature dependence of reaction rates is often explained by the Arrhenius equation, which states that the rate constant (\(k\)) of a reaction increases exponentially with temperature (\(T\)). This relationship suggests that increasing the temperature accelerates the reaction rate.
Given that the reaction occurs at a significant rate only if the temperature of the reaction mixture is greater than 200°C, we can infer that the reaction likely has a high activation energy. Activation energy is the energy barrier that must be overcome for a reaction to proceed. If the activation energy is high, a significant amount of thermal energy (high temperature) is required to provide the reactant molecules with enough kinetic energy to overcome this barrier and initiate the reaction.
So, the correct answer is:
(C) The reaction has a high activation energy.
Questions
\(Cu_{(s)}+4HNO_{3}(aq)\rightarrow Cu(No_{3})_{2}(aq)+2No_{2(g)}+2H_{2}o_{(l)}\)
Each student in a class placed a \(2.00 \mathrm{~g}\) sample of a mixture of \(\mathrm{Cu}\) and \(\mathrm{Al}\) in a beaker and placed the beaker in a fume hood. The students slowly poured \(15.0 \mathrm{~mL}\) of \(15.8 \mathrm{M} \mathrm{HNO}_3(a q)\) into their beakers. The reaction between the copper in the mixture and the \(\mathrm{HNO}_3(a q)\) is represented by the equation above. The students observed that a brown gas was released from the beakers and that the solutions turned blue, indicating the formation of \(\mathrm{Cu}^{2+}(a q)\). The solutions were then diluted with distilled water to known volumes.
In one student’s experiment the reaction proceeded at a much slower rate than it did in the other students’ experiments. Which of the following could explain the slower reaction rate?
(A) In the student’s sample the metal pieces were much smaller than those in the other students’ samples.
(B) The student heated the reaction mixture as the \(HNo_{3}(aq)\) was added.
(C) The student used a 1.5 M solution of \(HNo_{3}(aq)\) instead of a 15.8 M solution of \(HNo_{3}(aq)\).
(D) The student used a 3.00 g sample of the mixture instead of the 2.00 g sample that was used by the other students.
▶️Answer/Explanation
Ans: C
The slower reaction rate observed by the student could be explained by several factors.
(A) In the student’s sample, the metal pieces were much smaller than those in the other students’ samples.
Smaller metal pieces would increase the surface area of the metal exposed to the acid, leading to a faster reaction rate rather than a slower one. This option is unlikely to explain the slower reaction rate.
(B) The student heated the reaction mixture as the \(HNO_{3}(aq)\) was added.
Heating the reaction mixture would increase the kinetic energy of the particles, leading to more frequent and energetic collisions between the reactants. This would generally result in a faster reaction rate rather than a slower one.
(C) The student used a 1.5 M solution of \(HNO_{3}(aq)\) instead of a 15.8 M solution of \(HNO_{3}(aq)\).
A lower concentration of \(HNO_{3}(aq)\) would result in fewer collisions between the acid molecules and the metal, which could lead to a slower reaction rate. This option is plausible and could explain the slower reaction rate observed by the student.
(D) The student used a 3.00 g sample of the mixture instead of the 2.00 g sample that was used by the other students.
Using a larger sample of the mixture would result in more metal atoms available for reaction with the acid, potentially leading to a faster reaction rate rather than a slower one.
Based on the analysis, option (C) seems to be the most plausible explanation for the slower reaction rate observed by the student. Therefore, the correct answer is (C) The student used a 1.5 M solution of \(HNO_{3}(aq)\) instead of a 15.8 M solution of \(HNO_{3}(aq)\).
Question
\(C_{12}H_{22}O_{11}(aq)+H_2O(l)→2C_6H_{12}O_6(aq)\)
The chemical equation shown above represents the hydrolysis of sucrose. Under certain conditions, the rate is directly proportional to the concentration of sucrose. Which statement supports how a change in conditions can increase the rate of this reaction?
A Increasing the amount of water in which the sugar is dissolved will increase the frequency of collisions between the sucrose molecules and the water molecules resulting in an increase in the rate of hydrolysis.
B Decreasing the temperature will increase the frequency of the collisions between the sucrose molecules and the water molecules resulting in an increase in the rate of hydrolysis.
C Increasing the concentration of sucrose will increase the rate of hydrolysis by increasing the frequency of the collisions between the sucrose and the water molecules.
D Decreasing the concentration of sucrose will increase the rate of hydrolysis by increasing the frequency of the collisions between the sucrose and the water molecules.
▶️Answer/Explanation
Ans: C
The rate changes proportionally to the change in the concentration of sucrose and the increase in the rate of hydrolysis can be explained by an increase in the frequency of the collisions between the reactant molecules.
Question
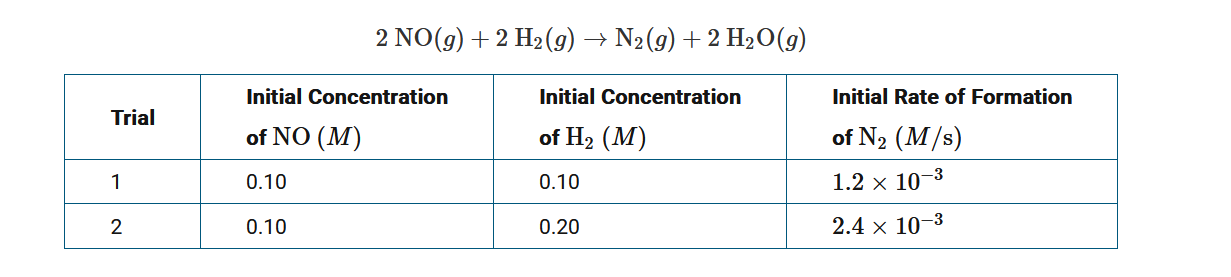
The information in the data table above represents two different trials for an experiment to study the rate of the reaction between NO(g) and \(H_2\)(g), as represented by the balanced equation above the table. Which of the following statements provides the correct explanation for why the initial rate of formation of \(N_2\) is greater in trial 2 than in trial 1? Assume that each trial is carried out at the same constant temperature.
A The activation energy of the reaction is smaller in trial 2 than it is in trial 1.
B The frequency of collisions between reactant molecules is greater in trial 2 than it is in trial 1.
C The value of the rate constant for the reaction is smaller in trial 2 than it is in trial 1.
D The value of the rate constant for the reaction is greater in trial 2 than it is in trial 1.
▶️Answer/Explanation
Ans: B
The concentration of H2 in trial 2 has been doubled. As reactant concentration increases, the frequency of collisions between reactant molecules increases. This leads to an increase in reaction rate.
Question
\(Zn(s)+2 HCl(aq)→ZnCl_2(aq)+H_2(g)\)
Zn(s) reacts with HCl(aq) according to the equation shown above. In trial 1 of a kinetics experiment, a 5.0g piece of Zn(s) is added to 100mL of 0.10MHCl(aq). The rate of reaction between Zn(s) and HCl(aq) is determined by measuring the volume of H2(g) produced over time. In trial 2 of the experiment, 5.0g of powdered Zn(s) is added to 100mL of 0.10M HCl(aq). Which trial will have a faster initial rate of reaction and why?
A Trial 1, because there is a higher concentration of Zn(s) in the reaction mixture.
B Trial 1, because the sample of Zn(s)has less surface area for the reaction to take place.
C Trial 2, because there is a higher concentration of HCl(aq) in the reaction mixture.
D Trial 2, because the sample of Zn(s) has a greater surface area for the reaction to take place.
▶️Answer/Explanation
Ans:D
The sample of powdered Zn(s) in trial 2 has a greater surface area than the piece of Zn(s) in trial 1; therefore, more Zn atoms are exposed to HCl(aq) in trial 2 than in trial 1, leading to a faster initial reaction rate.
Question
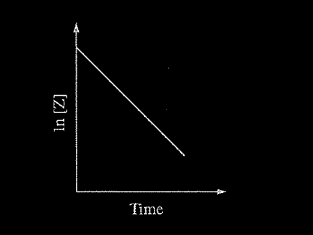
Consider the reaction represented by the equation \(2X + 2Z → X_2Z_2\). During a reaction in which a large excess of reactant X was present, the concentration of reactant Z was monitored over time. A plot of the natural logarithm of the concentration of Z versus time is shown in the figure above. The order of the reaction with respect to reactant Z is
(A) zero order
(B) first order
(C) second order
(D) third order
▶️Answer/Explanation
Ans:B