Question
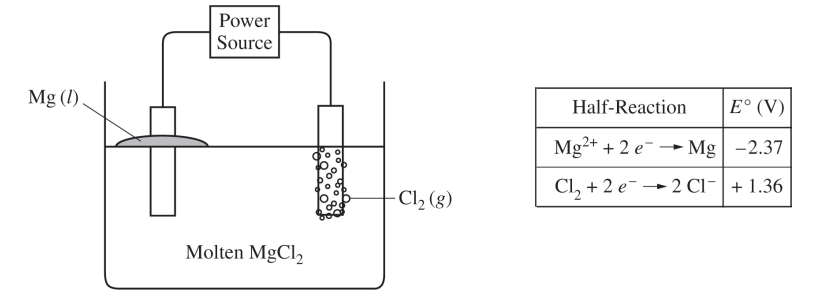
Molten MgCl2 can be decomposed into its elements if a sufficient voltage is applied using inert electrodes. The products of the reaction are liquid Mg (at the cathode) and Cl2 gas (at the anode). A simplified representation of the cell is shown above. The reduction half-reactions related to the overall reaction in the cell are given in the table.
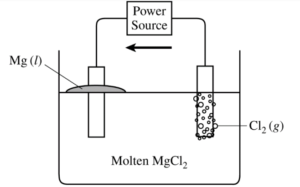
(a) Draw an arrow on the diagram to show the direction of electron flow through the external circuit as the cell operates.
(b) Would an applied voltage of 2.0 V be sufficient for the reaction to occur? Support your claim with a calculation as part of your answer.
(c) If the current in the cell is kept at a constant 5.00 amps, how many seconds does it take to produce 2.00 g of Mg(l) at the cathode?
▶️Answer/Explanation
Ans:
(a) For the correct answer:
Electron flow should be indicated only in a counter-clockwise direction in the external circuit, from the Cl2 anode to the Mg cathode.
(b) For the correct answer and calculated value:
No, because 2.0 V is less than 3.73 V, which is the minimum voltage needed for electrolysis to occur.
E0cell =− 2.37 V + ( -1.36 V) = -3.73 V
(c) For the correct calculated value of moles of electrons (may be implicit):
\(2.00 g Mg\times \frac{1 mol Mg}{24.30 g Mg}\times \frac{2 mol e^{-}}{1 mol Mg}=0.165 mol e^{-}\)
For the correct calculated number of seconds:
\(0.165 mol e^{-}\times \frac{96,485 C}{1 mol e^{-}}\times \frac{1s}{5.00 C}=3180 s\)
Question
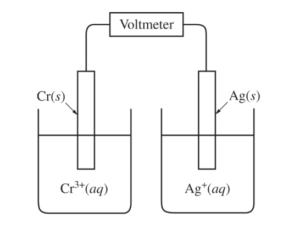
A student sets up a galvanic cell at 298 K that has an electrode of Ag(s) immersed in a 1.0 M solution of Ag +(aq) and an electrode of Cr(s) immersed in a 1.0 M solution of Cr3+(aq), as shown in the diagram above.
(a) The student measures the voltage of the cell shown above and discovers that it is zero. Identify the missing component of the cell, and explain its importance for obtaining a nonzero voltage.

(b) The student adds the missing component to the cell and measures E0cell to be +1.54 V. As the cell operates, Ag+ ions are reduced. Use this information and the information in the table above to do the following.
(i) Calculate the value of E0 for the half-reaction Cr 3+(aq) + 3 e− → Cr(s).
(ii) Write the balanced net-ionic equation for the overall reaction that occurs as the cell operates.
(iii) Calculate the value of ΔG° for the overall cell reaction in J/molrxn.
▶️Answer/Explanation
Ans:
(a)
| The salt bridge is missing. The salt bridge allows for the migration of ions to maintain charge balance in each half-cell. |
(b) (i)
E0cell = E0red (cathode) – E0red (anode) + 1.54 V = +0.80 V – x x = +0.80 V – (+1.54 V) = -0.74 V |
(ii)
| 3 Ag+(aq) + Cr(s) → 3 Ag(s) + Cr3+(aq) |
(iii)
\(\Delta G^{0}= – nFE^{0}=-\left ( \frac{3 mol e^{-}}{1 mol _{rxn}} \right )\left ( 96,485\frac{C}{mol e^{-}} \right )\left ( 1.54\frac{J}{C} \right )\) = -4.46 × 105 J/molrxn |
Question
A student wants to determine the concentration of H2O2 in a solution of H2O2(aq). The student can use one of two titrants, either dichromate ion, Cr2O72–(aq), or cobalt(II) ion, Co2+(aq). The balanced chemical equations for the two titration reactions are shown below.
Dichromate as titrant: Cr2O72–(aq) + 3 H2O2(aq) + 8 H+(aq) → 2 Cr3+(aq) + 3 O2(g) + 7 H2O(l)
Cobalt(II) as titrant: 2 Co2+(aq) + H2O2(aq) + 2 H+(aq) → 2 Co3+(aq) + 2 H2O(l)
The half-reactions and the E° values for the systems related to the titrations above are given in the following table.
(a) Use the information in the table to calculate the following.
(i) E° for the reaction between Cr2O72–(aq) and H2O2(aq) at 298 K
(ii) E° for the reaction between Co2+(aq) and H2O2(aq) at 298 K
(b) Based on the calculated values of E°, the student must choose the titrant for which the titration reaction is thermodynamically favorable at 298 K.
(i) Which titrant should the student choose? Explain your reasoning.
(ii) Calculate the value of ΔG°, in kJ/molrxn, for the reaction between the chosen titrant and H2O2(aq).
▶️Answer/Explanation
Ans:
(a) (i)
| E0 = 1.33 – 0.70 = 0.63 V |
(ii)
| E0 = -1.84 + 1.77 = -0.07 V |
(b) (i)
| The student should be the dichromate ion for the titration because, for the reaction, the value of E0 is positive, which means that the reaction is thermodynamically favorable. OR ΔG0 = -n FE0 and n, F, and E0 are all positive numbers, therefore ΔG0 < 0, which means that the reaction is thermodynamically favorable. |
(ii)
| \(\Delta G^{0}= – nFE^{0}= – 6(96,485\frac{C}{mol})(0.63\frac{J}{C})(\frac{1 kJ}{1000 J})=-360 kJ/mol_{rxn}\) |
