Questions

The table above provides some information about two types of steel, both of which are alloys of iron and carbon. Which of the following best helps to explain why high-carbon steel is more rigid than low-carbon steel?
(A) Elemental carbon is harder than elemental iron.
(B) The additional carbon atoms within the alloy make the high-carbon steel less dense.
(C) The additional carbon atoms within the alloy increase the thermal conductivity of the high-carbon steel.
(D) The additional carbon atoms within the alloy make it more difficult for the iron atoms to slide past one another.
▶️Answer/Explanation
Ans: D
The rigidity of steel is primarily influenced by its structure and composition. In this case, the difference between low-carbon steel and high-carbon steel lies in the amount of carbon present in the alloy.
Option (D) best explains why high-carbon steel is more rigid than low-carbon steel. The additional carbon atoms within the alloy make it more difficult for the iron atoms to slide past one another. This results in a stronger and less ductile structure, leading to increased rigidity. In high-carbon steel, the carbon atoms occupy the interstitial spaces between the iron atoms, disrupting the regular arrangement and making it harder for the iron atoms to move relative to each other. This increased resistance to movement contributes to the steel’s rigidity.
Options (A), (B), and (C) are not directly related to the increased rigidity of high-carbon steel compared to low-carbon steel. While elemental carbon is indeed harder than elemental iron (option A), the hardness of the alloy is more influenced by the arrangement of carbon atoms within the steel matrix. Option (B) about density is not relevant to the rigidity of the steel, and option (C) about thermal conductivity is also not directly related to rigidity.
Question
Which of the following is a formula for an ether?
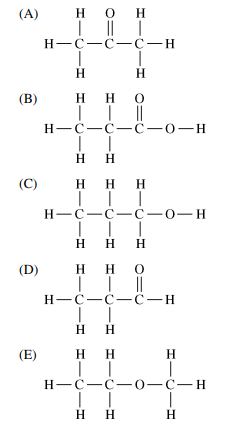
▶️Answer/Explanation
Ans:E
Question
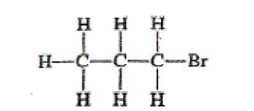
Which of the following structural formulas represents an isomer of the compound that has the structural formula represented above?
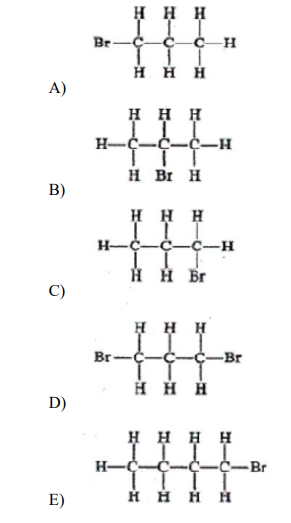
▶️Answer/Explanation
Ans:B
An isomer has the same chemical formula, but a different structural formula. Remember, these are not flat structures and since all singly bonded, the carbons can rotate around the central axis of the chain. In other words, if the Br is attached to either end of a 3-C chain, it is the same structure as presented and NOT an isomer. (E) is a 4-C chain and (A), (C), and (D) all have Br attached to an end carbon. Only answer (B) is an isomer.
DIF: Hard TOP: Bonding & Molecular Structure MSC: 2002 #62 NOT: 28% answered correctly
Question
Which of the following pairs of compounds are isomers?
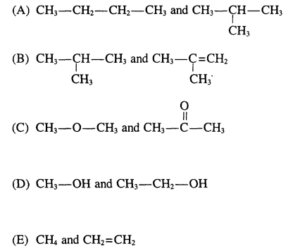
▶️Answer/Explanation
Ans: A