Question
A student carefully drops a 9.0g solid Zn pellet initially at 50.0°C into an insulated cup containing 30.0g of water at 27.8°C .The student predicts that the temperature of the water will increase after the pellet is added. Which of the following statements is the best justification for the student’s prediction?
A The metallic bonds between Zn atoms will break when the Zn is exposed to the water molecules, releasing energy that will be absorbed by the water molecules.
B Collisions between the water molecules and the surface of the Zn pellet will result in the transfer of energy, increasing the average kinetic energy of the water molecules.
C The strength of the hydrogen bonds between the water molecules will increase when the Zn pellet is added, decreasing the average kinetic energy of the water molecules.
D Collisions between Zn atoms in the solid will increase in frequency when the Zn is exposed to the water molecules, resulting in the transfer of energy to the surroundings.
▶️Answer/Explanation
Ans: B
Heat will be transferred from the Zn to the water through particle collisions, increasing the average kinetic energy of the water molecules and the temperature of the water.
Question
Two aqueous NaCl solutions of equal volume and concentration were kept in flasks and held at different temperatures. The two solutions were combined in a larger flask. Based on this information, which of the following predictions is correct?
A The average kinetic energy of the particles in the cooler solution will decrease as they collide with the particles from the warmer solution.
B The average kinetic energy of the particles in the cooler solution will increase as they collide with the particles from the warmer solution.
C The particles from the cooler solution and the particles from the warmer solution will reach thermal equilibrium through collisions, at which time the average kinetic energy of the particles in the mixture will be lower than the average kinetic energy that the particles had in the cooler solution.
D The particles from the cooler solution and the particles from the warmer solution will reach thermal equilibrium through collisions, at which time the average kinetic energy of the particles in the mixture will be higher than the average kinetic energy that the particles had in the warmer solution.
▶️Answer/Explanation
Ans: B
The average kinetic energy of the particles in the warmer solution is higher than the average kinetic energy of the particles in the cooler solution. Therefore, energy will be transferred from the particles in the warmer solution to the particles in the cooler solution through particle collisions.
Question
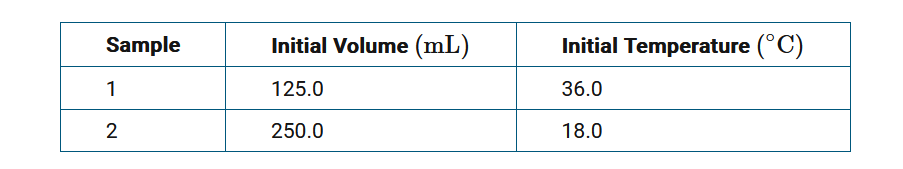
The table provides data for two \(CH_3OH(l)\) samples. Based on this information, which of the following statements describes what happens when these samples are initially mixed, and why?
A Thermal energy is transferred between the \(CH_3OH(l)\) molecules in sample 1 and the \(CH_3OH(l)\) molecules in sample 2 because both samples contain the same substance.
B The\(CH_3OH(l)\) molecules from sample 2 transfer thermal energy to the \(CH_3OH(l)\) molecules from sample 1 through collisions because there are more moles of molecules in sample 2.
C The \(CH_3OH(l)\) molecules from sample 1 transfer thermal energy to the \(CH_3OH(l)\) molecules from sample 2 through collisions because sample 1 has a higher density.
D The \(CH_3OH(l)\) molecules from sample 1 transfer thermal energy to the \(CH_3OH(l)\) molecules from sample 2 through collisions because the average kinetic energy of the molecules in sample 1 is greater.
▶️Answer/Explanation
Ans:D
The higher temperature of sample 1 indicates that the molecules in this sample have a greater average kinetic energy that can be transferred to the lower-energy molecules in sample 2.
Question
A 10. g cube of copper at a temperature \(T_1\) is placed in an insulated cup containing 10. g of water at a temperature \(T_2\). If T, > \(T_2\), which of the following is true of the system when it has attained thermal equilibrium? (The specific heat of copper is 0.385 J/(g-°C) and the specific heat of water is 4.18 J/(g.°C).)
(A) The temperature of the copper changed more than the temperature of the water.
(B) The temperature of the water changed more than the temperature of the copper.
(C) The temperature of the water and the copper changed by the same amount.
(D) The relative temperature changes of the copper and the water cannot be determined without knowing\( T_1\), and \(T_2\).
▶️Answer/Explanation
Ans:A
Question
Refer to the following graph, which shows the heating curve for methane, \(CH_4\)
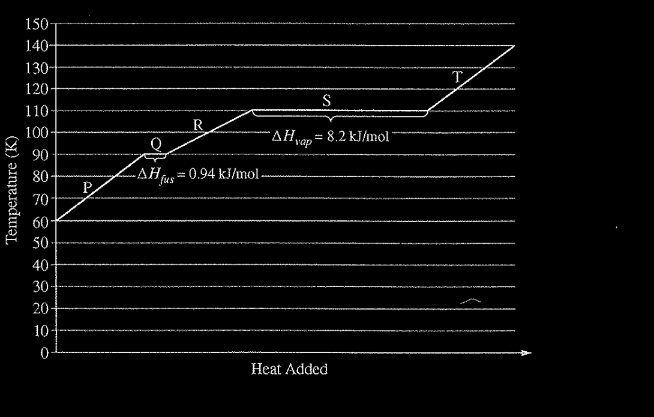
Which of the following best explains why more energy is required for the process occurring at 110 K than for the process occurring at 90 K ?
(A) Intermolecular attractions are completely overcome during vaporization.
(B) Intermolecular attractions in the solid phase are weaker than in the liquid phase.
(C) Electron clouds of methane molecules are less polarizable at lower temperatures.
(D) Vaporization involves a large increase in temperature.
▶️Answer/Explanation
Ans:A
