Question
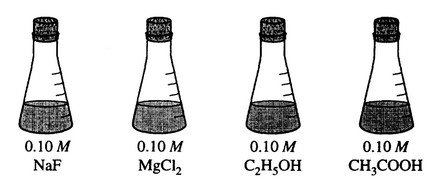
Answer the following questions, which refer to the 100 mL samples of aqueous solutions at \(25^°\)C in the stoppered flasks shown above.
(a) Which solution has the lowest electrical conductivity? Explain.
(b) Which solution has the lowest freezing point? Explain.
(c) Above which solution is the pressure of water vapor greatest? Explain.
(d) Which solution has the highest pH? Explain.
▶️Answer/Explanation
Answer:
(a) \(C_2H_5OH\) (Flask #3)
Ethanol, a nonelectrolyte, does not break up or dissociate in solution.
- One point earnedfor identifying \(C_2H_5OH\)
- One point earnedfor the correct explanation.
- Explanation point earnedfor a description of a nonelectrolyte (e.g., something
that does not break up or does not dissociate.) - No point earnedfor describing \(C_2H_5OH\) as organic, or as the compound that
contains the most hydrogens.
(b) \(MgCl_2\) (Flask #2)
The freezing-point depression is proportional to the concentration of solute particles.
All solutes are at the same concentration, but the van’t Hoff factor (i) is largest
for \(MgCl_2\).
- One point earned for identifying \(MgCI_2\) .
- One point earned for the correct explanation.
(c) \(C_2H_5OH\) (Flask #3)
The lowering of vapor pressure of water is directly proportional to the concentration
of solute particles in solution. \(C_2H_5OH\) is the only nonelectrolyte, so it will have the
fewest solute particles in solution.
- One point earnedfor identlfiing \(C_2H_5OH\).
- One point earnedfor the correct explanation.
(d) NaF (Flask #1)
The \(F^-\) ion, generated upon dissolution of NaF, is a weak base.
It is the only solution with pH > 7.
- One point earnedfor identlBing NaF.
- One point earnedfor the correct explanation.
Question
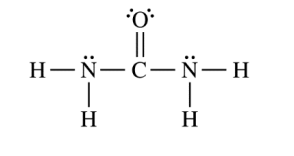
The compound urea, H2NCONH2 , is widely used in chemical fertilizers. The complete Lewis electron-dot diagram for the urea molecule is shown above.
(a) Identify the hybridization of the valence orbitals of the carbon atom in the urea molecule.
(b) Urea has a high solubility in water, due in part to its ability to form hydrogen bonds. A urea molecule and four water molecules are represented in the box below. Draw ONE dashed line (—-) to indicate a possible location of a hydrogen bond between a water molecule and the urea molecule.

The dissolution of urea is represented by the equation above. A student determines that 5.39 grams of H2NCONH2 (molar mass 60.06 g/mol) can dissolve in water to make 5.00 mL of a saturated solution at 20.°C.
(c) Calculate the concentration of urea, in mol/L, in the saturated solution at 20.°C.
(d) The student also determines that the concentration of urea in a saturated solution at 25°C is 19.8 M. Based on this information, is the dissolution of urea endothermic or exothermic? Justify your answer in terms of Le Chatelier’s principle.
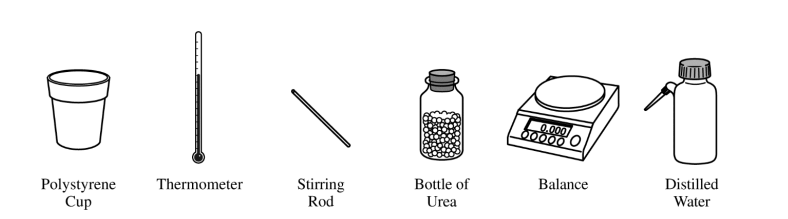
(e) The equipment shown above is provided so that the student can determine the value of the molar heat of solution for urea. Knowing that the specific heat of the solution is 4.18 J/(g⋅°C), list the specific measurements that are required to be made during the experiment.
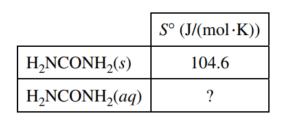
(f) The entropy change for the dissolution of urea, ΔS0soln , is 70.1 J/(mol⋅K) at 25°C. Using the information in the table above, calculate the absolute molar entropy, S°, of aqueous urea.
(g) Using particle-level reasoning, explain why ΔS0soln is positive for the dissolution of urea in water.
(h) The student claims that ΔS° for the process contributes to the thermodynamic favorability of the dissolution of urea at 25°C. Use the thermodynamic information above to support the student’s claim.
▶️Answer/Explanation
Ans:
(a)
| sp2 |
(b)
| A dashed line should connect a hydrogen atom in water to a nitrogen or oxygen atom in urea or an oxygen atom in water to a hydrogen atom in urea. One possible correct response is shown above. |
(c)
5.39 g H2NCONH2 × \(\frac{1 mol}{60.06 g}=0.0897 mol\) \(\frac{0.0897 mol}{0.00500 L}=17.9 M\) |
(d)
| The increased solubility at the higher temperature implies that the dissolution of urea is endothermic. If a saturated solution of urea is heated, then the equilibrium system is stressed. The stress is counteracted by the endothermic dissolution of more urea. |
(e)
| mass of urea, mass of water, initial temperature of water, final temperature of solution |
(f)
ΔS0soln = S0 (H2NCONH2(aq)) – S0 (H2NCONH2(s)) 70.1 J/(mol.K) = S0 (H2NCONH2(aq)) – 104.6 J/(mol.K) S0 (H2NCONH2(aq)) = 174.7 J/(mol.K) |
(g)
| Urea molecules in solution have a greater number of possible arrangements than in solid urea. This increased number of arrangements corresponds to a positive ΔS0soln. |
(h)
| Thermodynamic favorability for a process at standard conditions is determined by the sign of ΔG0 , with ΔG0 =ΔH0 -TΔS0 . Since ΔS0 is positive, the TΔS0 term makes the value of ΔG0 smaller and thus makes the dissolution more thermodynamically favorable. |
Question
NaHCO3(s) + HC2H3O2(aq) → NaC2H3O2(aq) + H2O(l) + CO2(g)
A student designs an experiment to study the reaction between NaHCO3 and HC2H3O2 . The reaction is represented by the equation above. The student places 2.24 g of NaHCO3 in a flask and adds 60.0 mL of 0.875 M HC2H3O2 . The student observes the formation of bubbles and that the flask gets cooler as the reaction proceeds.
(a) Identify the reaction represented above as an acid-base reaction, precipitation reaction, or redox reaction. Justify your answer.
(b) Based on the information above, identify the limiting reactant. Justify your answer with calculations.
(c) The student observes that the bubbling is rapid at the beginning of the reaction and gradually slows as the reaction continues. Explain this change in the reaction rate in terms of the collisions between reactant particles.
(d) In thermodynamic terms, a reaction can be driven by enthalpy, entropy, or both.
(i) Considering that the flask gets cooler as the reaction proceeds, what drives the chemical reaction between NaHCO3(s) and HC2H3O2(aq) ? Answer by drawing a circle around one of the choices below.
Enthalpy only Entropy only Both enthalpy and entropy
(ii) Justify your selection in part (d)(i) in terms of ΔG°.
(e) The HCO3− ion has three carbon-to-oxygen bonds. Two of the carbon-to-oxygen bonds have the same length and the third carbon-to-oxygen bond is longer than the other two. The hydrogen atom is bonded to one of the oxygen atoms. In the box below, draw a Lewis electron-dot diagram (or diagrams) for the HCO3− ion that is (are) consistent with the given information.

(f) A student prepares a solution containing equimolar amounts of HC2H3O2 and NaC2H3O2 . The pH of the solution is measured to be 4.7. The student adds two drops of 3.0 M HNO3(aq) and stirs the sample, observing that the pH remains at 4.7. Write a balanced, net-ionic equation for the reaction between HNO3(aq) and the chemical species in the sample that is responsible for the pH remaining at 4.7.
▶️Answer/Explanation
Ans:
(a)
| It is an acid-base reaction. The weak acid HC2H3O2 reacts with the weak base HCO3– with HC2H3O2 donating a proton. OR It is an acid-base reaction. No solid precipitates, so it is not a precipitation reaction. None of the oxidation numbers change, so it is not a redox reaction. |
(b)
\(2.24 g NaHCO_{3}\times \frac{1 mol NaHCO_{3}}{84.0 g}= 0.0267 mol NaHCO_{3}\) \(60.0 mL\times \frac{0.875 mol HC_{2}H_{3}O_{2}}{1000 mL}= 0.0525 mol HC_{2}H_{3}O_{2}\) The NaHCO3(s) and HC2H3O2 (aq) react in a 1:1 ratio, so the limiting reactant is NaHCO3(s). |
(c)
| As the reaction proceeds, both reactants are consumed and their concentrations decrease. Collisions between reactant particles become less likely as their concentrations decrease, thus the reaction rate slows. |
(d) (i)
| Entropy only should be circled. |
(ii)
ΔG0 = ΔH0 – TΔS0 Reactions are thermodynamically favorable when ΔG0 is negative. Since the reaction is endothermic (the flask cooler, ΔH0 is positive), the reaction is not driven by enthalpy, because enthalpy does not help make ΔG0 negative. Because there are no gases in the reactants and one of the products is a gas, ΔS0 must be positive, which helps make ΔG0 negative. |
(e)

| See diagram above. |
(f)
| C2H3O2– + H3O+ → HC2H3O2 + H2O OR C2H3O2– + H+ → HC2H3O2 |
