Question
Answer the following questions relating to HCl,\(CH_3Cl,\) and \(CH_3\)Br.
(a) HCl(g) can be prepared by the reaction of concentrated \(H_ 2SO_4\)(aq) with NaCl(s), as represented by the following equation.
\(H_{2}SO_{4}(aq)+2NaCl(s)\rightarrow 2HCl(g)+Na_{2}SO_{4}(aq)\)
(i) A student claims that the reaction is a redox reaction. Is the student correct? Justify your answer.
(ii) Calculate the mass, in grams, of NaCl(s) needed to react with excess \(H_2SO_4\)(aq) to produce 3.00 g of HCl(g). Assume that the reaction goes to completion.HCl(g) can react with methanol vapor,\( CH_3OH(g)\), to produce \(CH_3Cl\)(g), as represented by the following equation.
\(CH_{3}OH(g)+HCl(g)\rightleftharpoons CH_{3}Cl(g)+H_{2}O(g) K_{P}=4.7\times 10^{3}\) At400K
(b) \(CH _3OH(g)\) and HCl(g) are combined in a 10.00 L sealed reaction vessel and allowed to reach equilibrium at 400 K. The initial partial pressure of\( CH_3OH(g)\) in the vessel is 0.250 atm and that of HCl(g) is 0.600 atm.
(i) Does the total pressure in the vessel increase, decrease, or remain the same as equilibrium is approached? Justify your answer in terms of the reaction stoichiometry.
(ii) Considering the value of Kp , calculate the final partial pressure of HCl(g) after the system inside the vessel reaches equilibrium at 400 K.
(iii) The student claims that the final partial pressure of \(CH_3OH(g) \)at equilibrium is very small but not exactly zero. Do you agree or disagree with the student’s claim? Justify your answer.
(c) The table below shows some data for the compounds \(CH _3Cl \)and \(CH_3Br\).
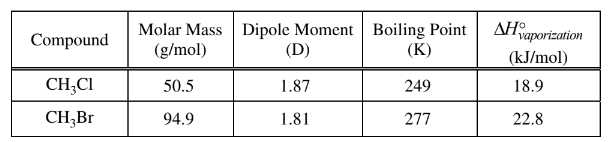
(i) Identify all the types of intermolecular forces that exist among molecules in \(CH_3Cl(l)\).
(ii) In terms of intermolecular forces, explain why the boiling point of CH3Br(l) is greater than that of \(CH_3Cl(l)\).
(d) A 2.00 mL sealed glass vial containing a 1.00 g sample of \(CH_3Cl(l)\) is stored in a freezer at 233 K.
(i) Calculate the pressure in the vial at 298 K assuming that all the \(CH_3Cl(l)\)vaporizes.
(ii) Explain why it would be unsafe to remove the vial from the freezer and leave it on a lab bench at 298 K.
▶️Answer/Explanation
a(i) No, the student is not correct. None of the oxidation numbers of the elements change (H = +1, S = +6, O = -2, Na = +1, Cl = -1).
a(ii) 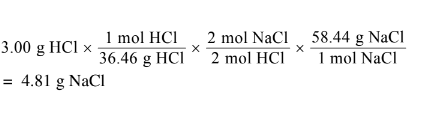
(b)(i) The pressure will remain the same. The reaction stoichiometry shows that two moles of gaseous reactants produce two moles of gaseous products. Because the number of moles of gas does not change, the pressure does not change.
b(ii)The value of\( K_p\) is large, so the reaction will proceed to the right until the limiting reactant is essentially used up. Thus practically all of the \(CH_3OH(g)\) will react and the final pressure of HCl(g) is 0.600 – 0.250 = 0.350 atm.
OR
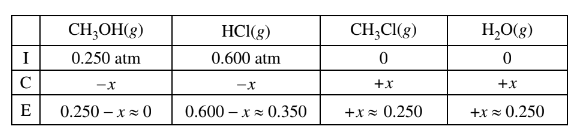
The final pressure of HCl(g) is 0.350 atm at equilibrium.
b(iii) Agree. The large value of \(K_p\) means that the partial pressure of the limiting reactant at equilibrium will be extremely small, but some \(CH_3OH \)molecules must exist for the system to be in dynamic equilibrium.
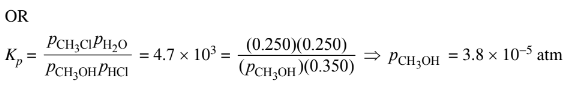
The partial pressure of \(CH_3OH(g)\) is very small but is not zero.
c(i) London dispersion forces and dipole-dipole forces
c(ii) The electron cloud in \(CH_3Br\) is larger and more polarizable than that of \(CH_3Cl\). As a result the London dispersion forces are stronger in \(CH_3Br\) compared to those in \(CH_3Cl\)and consequently the boiling point of\( CH_3\)Br is higher than that of \(CH_3Cl\).
d(i)
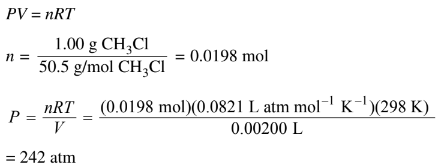
d(ii) At room temperature the liquid will vaporize. Consequently the glass vial may not be strong enough to withstand the increase in pressure.
Question
Na 2S2O3(aq) + 4 NaOCl(aq) + 2 NaOH(aq) → 2 Na2SO4(aq) + 4 NaCl(aq) + H2O(l)
A student performs an experiment to determine the value of the enthalpy change, ΔH0rxn , for the oxidation-reduction reaction represented by the balanced equation above.
(a) Determine the oxidation number of Cl in NaOCl.
(b) Calculate the number of grams of Na2S2O3 needed to prepare 100.00 mL of 0.500 M Na2S2O3 (aq).
In the experiment, the student uses the solutions shown in the table below.
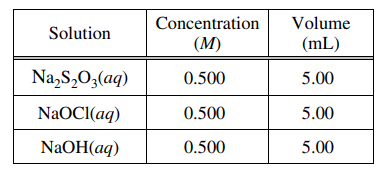
(c) Using the balanced equation for the oxidation-reduction reaction and the information in the table above,
determine which reactant is the limiting reactant. Justify your answer.
The solutions, all originally at 20.0 0C, are combined in an insulated calorimeter. The temperature of the reaction mixture is monitored, as shown in the graph below.
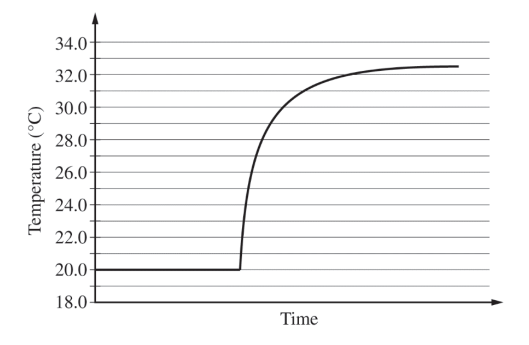
(d) According to the graph, what is the temperature change of the reaction mixture?
(e) The mass of the reaction mixture inside the calorimeter is 15.21 g.
(i) Calculate the magnitude of the heat energy, in joules, that is released during the reaction. Assume that the specific heat of the reaction mixture is 3.94 J/(g·°C) and that the heat absorbed by the calorimeter is negligible.
(ii) Using the balanced equation for the oxidation-reduction reaction and your answer to part (c), calculate D the value of the enthalpy change of the reaction, ΔH0rxn , in kJ/molrxn . Include the appropriate algebraic sign with your answer.
The student repeats the experiment, but this time doubling the volume of each of the reactants, as shown in the table below.
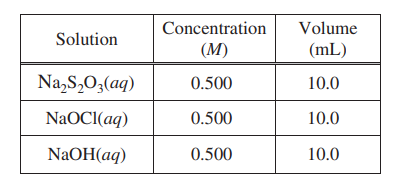
(f) The magnitude of the enthalpy change, ΔH0rxn, in kJ/molrxn , calculated from the results of the second
experiment is the same as the result calculated in part (e)(ii). Explain this result.
(g) Write the balanced net ionic equation for the given reaction.
▶️Answer/Explanation
Ans:
(a)
| +1 |
(b)
\(100.00mL\times \frac{0.500 mol Na_{2}S_{2}O_{3}}{1000 mL}\times \frac{158.10 g Na_{2}S_{2}O_{3}}{1 mol Na_{2}S_{2}O_{3}}\) = 7.90 g Na2S2O3 |
(c)
NaOCl is the limiting reactant. Given that equal numbers of moles of each reactant were present initially, it follows from the coefficients of the reactants in the balanced equation that NaOCl will be depleted first. |
(d)
| From the graph the final temperature is 32.50 C. ΔT = Tf – Ti = 32.50 C – 20.00 C = 12.50 C. |
(e) (i)
| q = mcΔT = (15.21 g) (3.94 J/(g.0C))(12.50 C) = 749 J |
(ii)
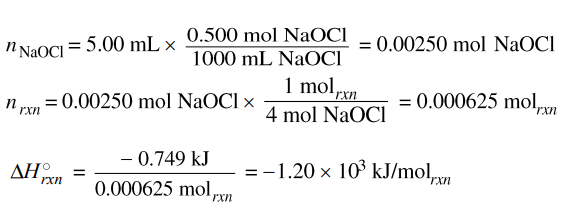 |
(f)
By doubling the volumes, the number of moles of the reactants are |
(g)
| S2O32-(aq) + 4OCl– (aq) + 2 OH–(aq) → 2SO42-(aq) + 4 Cl–(aq) + H2O(l) |
