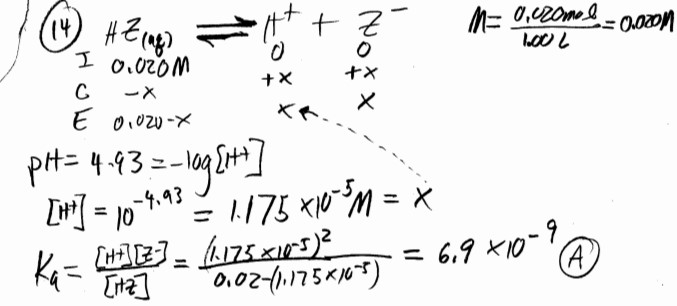Question
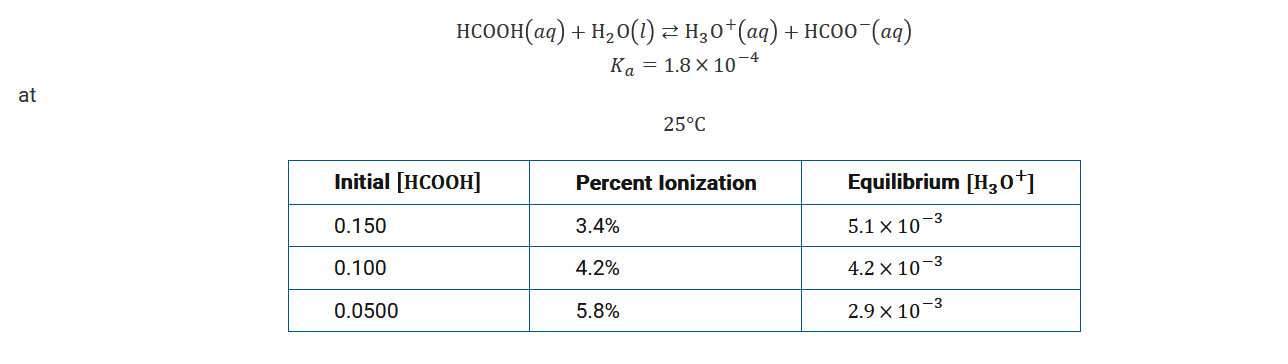
The equilibrium for the acid ionization of \(HCOOH\) is represented by the equation above, and the table gives the percent ionization for \(HCOOH\) at different initial concentrations of the weak acid at 25°C. Based on the information, which of the following is true for a 0.125M aqueous solution of \(HCOOH\)?
A It has a higher percent ionization, a lower \([H_3O^+]_{eq}\) , and a lower \(pH\) than a 0.150M \(HCOOH\) solution does.
B It has a higher percent ionization, a lower \([H_3O^+]_{eq}\) , and a higher \(pOH\) than a 0.150M \(HCOOH\)solution does.
C It has a lower percent ionization, a higher \([H_3O^+]_{eq}\) , and a higher \(pOH\) than a 0.100M \(HCOOH\) solution does.
D It has a lower percent ionization, a higher \([H_3O^+]_{eq}\) , and a higher \(pH\) than a 0.100M \(HCOOH\) solution does.
▶️Answer/Explanation
Ans:C
Based on the information given, when the initial concentration of the weak acid increases, the percent ionization decreases and the equilibrium concentration of \(H_3O^+\) increases. A higher \([H_3O^+]_{eq}\) results in a lower \([OH^−]_{eq}\) and a higher \(pOH\).
Question
A solution containing HCl and the weak acid\( HCIO_{2}\) has a pH of 2.4. Enough KOH(aq) is added to the solution to increase the pH to 10.5. The amount of which of the following species increases as the KOH(aq) is added?
(A) \(Cl^{-}\)(aq)
(B)\( H^{+}(aq)\)
(C) \(CIO_2^{-}\) (aq)
(D) \(HCIO_2\)(aq)
▶️Answer/Explanation
Ans:C
Question
Of the following, __________ is a weak acid.
A) HBr B) HF C) \(HClO_4\) D) HCl E) \(HNO_3\)
▶️Answer/Explanation
Ans: B
Question
Which one of the following is the weakest acid?
A) HF (\(K_a = 6.8 ̨\times 10^{-4}\))
B) Acetic acid (\(K_a = 1.8 ̨\times 10^{-5}\))
C) \(HNO_2 (K_a = 4.5 ̨\times 10^{-4})\)
D) HClO (\(K_a = 3.0 ̨\times 10^{-8})\)
E) HCN (\(K_a = 4.9 ̨\times 10^{-10}\))
▶️Answer/Explanation
Ans: E

Question
HZ is a weak acid. An aqueous solution of HZ is prepared by dissolving 0.020 mol of HZ in sufficient water to
yield 1.00 L of solution. The pH of the solution was 4.93 at \(25^o\)C. The \(K_a\) of HZ is __________.
A) \(6.9 ̨\times 10^{-9}\) B) \(1.4 ̨\times 10^{-10}\) C) \(1.2 ̨\times 10^{-5}\) D) \(2.8 ̨\times 10^{-1}\)2 E) \(9.9 ̨\times 10^{-2}\)
▶️Answer/Explanation
Ans: A