Question
Answer the following questions relating to HCl,\(CH_3Cl,\) and \(CH_3\)Br.
(a) HCl(g) can be prepared by the reaction of concentrated \(H_ 2SO_4\)(aq) with NaCl(s), as represented by the following equation.
\(H_{2}SO_{4}(aq)+2NaCl(s)\rightarrow 2HCl(g)+Na_{2}SO_{4}(aq)\)
(i) A student claims that the reaction is a redox reaction. Is the student correct? Justify your answer.
(ii) Calculate the mass, in grams, of NaCl(s) needed to react with excess \(H_2SO_4\)(aq) to produce 3.00 g of HCl(g). Assume that the reaction goes to completion.HCl(g) can react with methanol vapor,\( CH_3OH(g)\), to produce \(CH_3Cl\)(g), as represented by the following equation.
\(CH_{3}OH(g)+HCl(g)\rightleftharpoons CH_{3}Cl(g)+H_{2}O(g) K_{P}=4.7\times 10^{3}\) At400K
(b) \(CH _3OH(g)\) and HCl(g) are combined in a 10.00 L sealed reaction vessel and allowed to reach equilibrium at 400 K. The initial partial pressure of\( CH_3OH(g)\) in the vessel is 0.250 atm and that of HCl(g) is 0.600 atm.
(i) Does the total pressure in the vessel increase, decrease, or remain the same as equilibrium is approached? Justify your answer in terms of the reaction stoichiometry.
(ii) Considering the value of Kp , calculate the final partial pressure of HCl(g) after the system inside the vessel reaches equilibrium at 400 K.
(iii) The student claims that the final partial pressure of \(CH_3OH(g) \)at equilibrium is very small but not exactly zero. Do you agree or disagree with the student’s claim? Justify your answer.
(c) The table below shows some data for the compounds \(CH _3Cl \)and \(CH_3Br\).
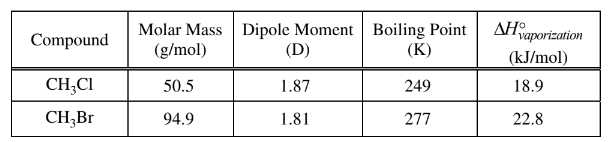
(i) Identify all the types of intermolecular forces that exist among molecules in \(CH_3Cl(l)\).
(ii) In terms of intermolecular forces, explain why the boiling point of CH3Br(l) is greater than that of \(CH_3Cl(l)\).
(d) A 2.00 mL sealed glass vial containing a 1.00 g sample of \(CH_3Cl(l)\) is stored in a freezer at 233 K.
(i) Calculate the pressure in the vial at 298 K assuming that all the \(CH_3Cl(l)\)vaporizes.
(ii) Explain why it would be unsafe to remove the vial from the freezer and leave it on a lab bench at 298 K.
▶️Answer/Explanation
a(i) No, the student is not correct. None of the oxidation numbers of the elements change (H = +1, S = +6, O = -2, Na = +1, Cl = -1).
a(ii) 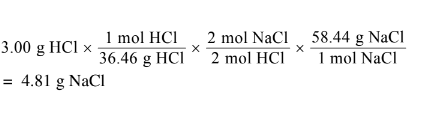
(b)(i) The pressure will remain the same. The reaction stoichiometry shows that two moles of gaseous reactants produce two moles of gaseous products. Because the number of moles of gas does not change, the pressure does not change.
b(ii)The value of\( K_p\) is large, so the reaction will proceed to the right until the limiting reactant is essentially used up. Thus practically all of the \(CH_3OH(g)\) will react and the final pressure of HCl(g) is 0.600 – 0.250 = 0.350 atm.
OR
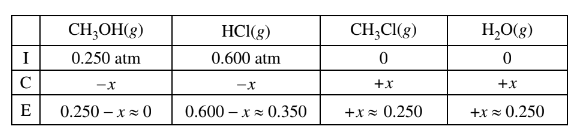
The final pressure of HCl(g) is 0.350 atm at equilibrium.
b(iii) Agree. The large value of \(K_p\) means that the partial pressure of the limiting reactant at equilibrium will be extremely small, but some \(CH_3OH \)molecules must exist for the system to be in dynamic equilibrium.
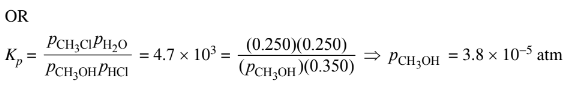
The partial pressure of \(CH_3OH(g)\) is very small but is not zero.
c(i) London dispersion forces and dipole-dipole forces
c(ii) The electron cloud in \(CH_3Br\) is larger and more polarizable than that of \(CH_3Cl\). As a result the London dispersion forces are stronger in \(CH_3Br\) compared to those in \(CH_3Cl\)and consequently the boiling point of\( CH_3\)Br is higher than that of \(CH_3Cl\).
d(i)
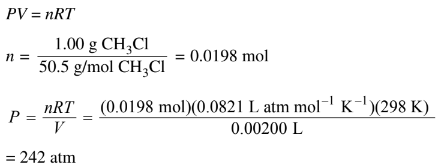
d(ii) At room temperature the liquid will vaporize. Consequently the glass vial may not be strong enough to withstand the increase in pressure.
Question
Answer the following questions relating to Fe and its ions, Fe2+ and Fe3+.
(a) Write the ground-state electron configuration of the Fe2+ ion.
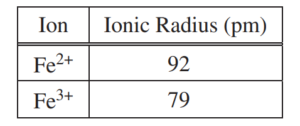
(b) The radii of the ions are given in the table above. Using principles of atomic structure, explain why the radius of the Fe2+ ion is larger than the radius of the Fe3+ ion.
(c) Fe3+ ions interact more strongly with water molecules in aqueous solution than Fe2+ ions do. Give one reason for this stronger interaction, and justify your answer using Coulomb’s law.
A student obtains a solution that contains an unknown concentration of Fe2+(aq). To determine the concentration
of Fe2+(aq) in the solution, the student titrates a sample of the solution with MnO4 –(aq) , which converts Fe2+(aq) to Fe3+(aq), as represented by the following equation.
5 Fe2+(aq) + MnO4−(aq) + 8 H+(aq) → 5 Fe3+(aq) + Mn2+(aq) + 4 H2O(l)
(d) Write the balanced equation for the half-reaction for the oxidation of Fe2+(aq) to Fe3+(aq).
(e) The student titrates a 10.0 mL sample of the Fe2+(aq) solution. Calculate the value of [Fe2+] in the solution if it takes 17.48 mL of added 0.0350 M KMnO4(aq) to reach the equivalence point of the titration.
To deliver the 10.0 mL sample of the Fe2+(aq) solution in part (e), the student has the choice of using one of the pieces of glassware listed below.
• 25 mL buret • 25 mL beaker
• 25 mL graduated cylinder • 25 mL volumetric flask
(f) Explain why the 25 mL volumetric flask would be a poor choice to use for delivering the required volume of the Fe2+(aq) solution.
In a separate experiment, the student is given a sample of powdered Fe(s) that contains an inert impurity. The student uses a procedure to oxidize the Fe(s) in the sample to Fe2O3(s). The student collects the following data during the experiment.
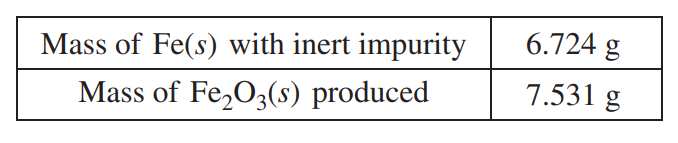
(g) Calculate the number of moles of Fe in the Fe2O3(s) produced.
(h) Calculate the percent by mass of Fe in the original sample of powdered Fe(s) with the inert impurity.
(i) If the oxidation of the Fe(s) in the original sample was incomplete so that some of the 7.531 g of product was FeO(s) instead of Fe2O3(s), would the calculated mass percent of Fe(s) in the original sample be higher, lower, or the same as the actual mass percent of Fe(s)? Justify your answer
▶️Answer/Explanation
Ans:
(a)
| 1s2 2s2 2p6 3s2 3p6 3d6 OR [Ar] 3d6 |
(b)
| Both ions have the same nuclear charge; however, the greater number of electrons in the outermost shell of Fe2+ results in greater electron – electron repulsion that shell, leading to a larger radius. |
(c)
| Coulomb’s law : \(F \propto \frac{q_{1}q_{2}}{r^{2}}\) (need not be explicitly stated) IN comparison to the Fe2+ ion, the Fe3+ ion has a higher charge. OR The smaller size of Fe3+ allows it to get closer to a water molecule. |
(d)
| Fe2+(aq) → Fe3+ (aq) + e– |
(e)
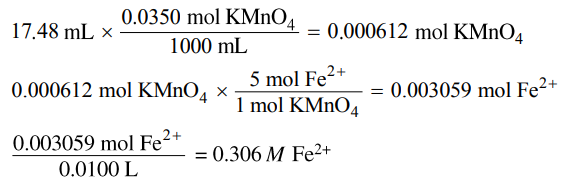 |
(f)
| The volumetric flask is designed to contain only 25.00 mL precisely. |
(g)
7.531 g Fe2O3 × \(\frac{1 mol Fe_{2}O_{3}}{159.70 g Fe_{2}O_{3}}\) = 0.04716 mol Fe2O3 0.04716 mol Fe2O3 × \(\frac{2 mol Fe}{1 mol Fe_{2}O_{3}}\) = 0.09431 mol Fe |
(h)
0.09431 mol Fe × \(\) = 5.267 g Fe \(\frac{5.267 g Fe}{6.724 g Sample}\times 100= 78.33%\) |
(i)
The calculated mass percent of Fe would be lower than the actual mass percent of Fe. A sample that contains any FeO (rather than Fe2O3) will have a higher actual mass percent of Fe than a completely oxidized sample would have. Therefore, when the moles of Fe are calculated (assuming all the mass of the sample is Fe2O3) the calculated number of moles of Fe, and hence the calculated mass percent of Fe, will be lower. |
Question
Answer the following questions relating to Fe and its ions, Fe2+ and Fe3+.
(a) Write the ground-state electron configuration of the Fe2+ ion.

(b) The radii of the ions are given in the table above. Using principles of atomic structure, explain why the radius of the Fe2+ ion is larger than the radius of the Fe3+ ion.
(c) Fe3+ ions interact more strongly with water molecules in aqueous solution than Fe2+ ions do. Give one reason for this stronger interaction, and justify your answer using Coulomb’s law.
A student obtains a solution that contains an unknown concentration of Fe2+(aq). To determine the concentration
of Fe2+(aq) in the solution, the student titrates a sample of the solution with MnO4 –(aq) , which converts Fe2+(aq) to Fe3+(aq), as represented by the following equation.
5 Fe2+(aq) + MnO4−(aq) + 8 H+(aq) → 5 Fe3+(aq) + Mn2+(aq) + 4 H2O(l)
(d) Write the balanced equation for the half-reaction for the oxidation of Fe2+(aq) to Fe3+(aq).
(e) The student titrates a 10.0 mL sample of the Fe2+(aq) solution. Calculate the value of [Fe2+] in the solution if it takes 17.48 mL of added 0.0350 M KMnO4(aq) to reach the equivalence point of the titration.
To deliver the 10.0 mL sample of the Fe2+(aq) solution in part (e), the student has the choice of using one of the pieces of glassware listed below.
• 25 mL buret • 25 mL beaker
• 25 mL graduated cylinder • 25 mL volumetric flask
(f) Explain why the 25 mL volumetric flask would be a poor choice to use for delivering the required volume of the Fe2+(aq) solution.
In a separate experiment, the student is given a sample of powdered Fe(s) that contains an inert impurity. The student uses a procedure to oxidize the Fe(s) in the sample to Fe2O3(s). The student collects the following data during the experiment.

(g) Calculate the number of moles of Fe in the Fe2O3(s) produced.
(h) Calculate the percent by mass of Fe in the original sample of powdered Fe(s) with the inert impurity.
(i) If the oxidation of the Fe(s) in the original sample was incomplete so that some of the 7.531 g of product was FeO(s) instead of Fe2O3(s), would the calculated mass percent of Fe(s) in the original sample be higher, lower, or the same as the actual mass percent of Fe(s)? Justify your answer
▶️Answer/Explanation
Ans:
(a)
| 1s2 2s2 2p6 3s2 3p6 3d6 OR [Ar] 3d6 |
(b)
| Both ions have the same nuclear charge; however, the greater number of electrons in the outermost shell of Fe2+ results in greater electron – electron repulsion that shell, leading to a larger radius. |
(c)
| Coulomb’s law : \(F \propto \frac{q_{1}q_{2}}{r^{2}}\) (need not be explicitly stated) IN comparison to the Fe2+ ion, the Fe3+ ion has a higher charge. OR The smaller size of Fe3+ allows it to get closer to a water molecule. |
(d)
| Fe2+(aq) → Fe3+ (aq) + e– |
(e)
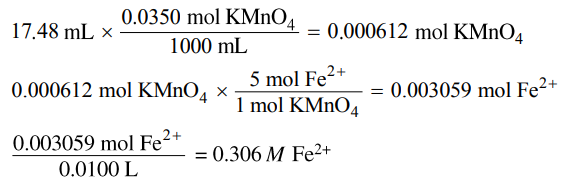 |
(f)
| The volumetric flask is designed to contain only 25.00 mL precisely. |
(g)
7.531 g Fe2O3 × \(\frac{1 mol Fe_{2}O_{3}}{159.70 g Fe_{2}O_{3}}\) = 0.04716 mol Fe2O3 0.04716 mol Fe2O3 × \(\frac{2 mol Fe}{1 mol Fe_{2}O_{3}}\) = 0.09431 mol Fe |
(h)
0.09431 mol Fe × \(\) = 5.267 g Fe \(\frac{5.267 g Fe}{6.724 g Sample}\times 100= 78.33%\) |
(i)
The calculated mass percent of Fe would be lower than the actual mass percent of Fe. A sample that contains any FeO (rather than Fe2O3) will have a higher actual mass percent of Fe than a completely oxidized sample would have. Therefore, when the moles of Fe are calculated (assuming all the mass of the sample is Fe2O3) the calculated number of moles of Fe, and hence the calculated mass percent of Fe, will be lower. |
Question
The boiling point of carbon tetrachloride (\(CCl_4\)) is higher than that of chloroform (\(CHCl_3\)). Since chloroform is
polar and carbon tetrachloride is not, consideration of dipole-dipole forces would predict that chloroform
would have the higher boiling point. How can we account for the observed order of the boiling points?
▶️Answer/Explanation
Ans:
Carbon tetrachloride is significantly larger than chloroform, and larger molecules tend to have greater polarizabilities
because they have a greater number of electrons and their electrons are further from the nuclei. Thus, London
dispersion forces between carbon tetrachloride molecules raises its boiling point above that of chloroform even though
chloroform experiences both London dispersion and dipole-dipole forces.
Ordinarily, and all things being equal, one would expect the following order of intermolecular forces:
H-bonding is stronger than dipole-dipole interactions, and dipole-dipole interactions are stronger than London
dispersion forces
H-bonding > dipole-dipole interactions > London dispersion forces
However, this problem states that the boiling point of the polar substance, \(CHCl_3\) is lower than that of the
nonpolar substance, \(CCl_4\).
This might not have been expected, but it is true. How could this be rationalized? Well, it must mean that the
London dispersion forces among \(CCl_4\) molecules is greater than the dipole-dipole interactions among \(CHCl_3\)
molecules. The permanent dipole of the chloroform molecules must not cause as great an effect as the
intermolecular attraction caused by the LDF among the carbon tetrachloride molecules. Carbon tetrachloride
has a total of 74 electrons which could possibly be temporarily unevenly distributed. \(CHCl_3\), however, only has
58 electrons. Apparently, the greater number of electrons in in \(CCl_4\) has a bigger effect on boiling point than the
permanent dipole of the \(CHCl_3\) molecule.
The hierarchy of intermolecular force strengths given above is only the usual and expected order of forces, all
things being equal. Note that water, for instance, is a very good example of H-bonding, but it is still a liquid at
room temperature. Elemental diatomic iodine (\(I_2\)), however, even though only held to other iodine molecules
through London dispersion forces, is a solid at room temperature, indicating that it has stronger intermolecular
forces than water does.
