Questions
Which of the following equations best represents the species that react and the species that are produced when \(CaCO_{3}\)(s) and HCl(aq) are combined?
(A)\( 2 H^{+}(aq) + CaCO_{3}(s) \rightarrow Ca^{2+}(aq) + CO_{3}^{2-}(aq) + H_{2}\)(g)
(B) \(2 H^{+}(aq) + CaCO_{3}(s) \rightarrow Ca^{2+}(aq) + H_2O(l) + CO_2(g)\)
(C) \(2 H^{+}(aq) + CaCO_3(s) \rightarrow Ca^{2+}(aq) + 2 OH^{-} (aq) + CO(g) \)
(D) \(2 HCl(aq) + CaCO_{3}(s) \rightarrow Ca^{2+}(aq) + H_2O(l) + CO_{2}(g) + 2 Cl^{-}\)(aq)
▶️Answer/Explanation
Ans: D
The correct equation that represents the reaction between \(CaCO_3\) (s) and HCI (aq) is:
\(2 HCl(aq) + CaCO_{3}(s) \rightarrow Ca^{2+}(aq) + H_2O(l) + CO_{2}(g) + 2 Cl^{-}\)(aq)
This equation shows that calcium carbonate reacts with hydrochloric acid to produce calcium ions, water, carbon dioxide gas, and chloride ions. The carbon dioxide gas produced is what causes the pressure
increase in the reaction vessel.
Questions
\(2H_{2(g)}+O_{2(g)}\rightarrow 2H_{2}O(g)\)
When \(H_{2(g)}\) and \(O_{2(g)}\) are mixed together in a rigid reaction vessel at 25\(^{\circ }C\), no reaction occurs. When the mixture is sparked, however, the gases react vigorously according to the equation above, releasing heat. Which of the following statements correctly explains why the spark is needed for the reaction to occur when the gases are originally at 25\(^{\circ }C\)?
(A) The reaction is not thermodynamically favorable at 25\(^{\circ }C\).
(B) \(\bigtriangleup H_{\circ }\) for the reaction has a large positive value at 25\(^{\circ }C\).
(C) \(\bigtriangleup S_{\circ }\) for the reaction has a large negative value at 25\(^{\circ }C\).
(D) The reaction has a large activation energy at 25\(^{\circ }C\).
▶️Answer/Explanation
Ans: D
The reaction described is the combustion of hydrogen gas (\(H_{2(g)}\)) with oxygen gas (\(O_{2(g)}\)) to form water vapor (\(H_{2}O(g)\)).
When \(H_{2(g)}\) and \(O_{2(g)}\) are mixed together at 25°C in a rigid reaction vessel, no reaction occurs. This suggests that the reaction does not occur spontaneously under those conditions, even though the reactants are present.
To initiate the reaction, a spark is needed. The spark provides the activation energy required for the reaction to proceed. Without the spark, the reactant molecules do not have sufficient energy to overcome the activation energy barrier and react.
Therefore, the correct statement to explain why the spark is needed for the reaction to occur at 25°C is: (D) The reaction has a large activation energy at 25°C.
Question
\( Zn(s)+2HCl(aq)→ZnCl_2(aq)+H_2(g)\)
When the reaction represented above proceeds, heat is produced. Which of the following best describes the reaction?
A It is a combustion reaction because heat is produced by the reaction.
B It is a double replacement reaction because 2Cl atoms are added to Zn.
C It is an acid-base reaction because HCl is an acid that is capable of exchanging \(H^+\).
D It is an oxidation-reduction reaction because zinc is oxidized and hydrogen is reduced.
▶️Answer/Explanation
Ans:D
It is an oxidation-reduction reaction because a Zn atom is oxidized and loses two electrons while two \(H^+\) ions are reduced as each gains an electron.
Question

Which of the following best describes the process represented above that takes place when \(NH_3\) is added to water?
A It is a single replacement reaction in which an electron pair on N is replaced with an H atom.
B It is an acid-base reaction in which a proton is exchanged from \(H_2O\) to \(NH_3\) .
C It is a precipitation reaction in which \(NH_4OH\) , an insoluble solid, is produced.
D It is an oxidation-reduction reaction in which the oxidation number of N changes from −3 to −4 .
▶️Answer/Explanation
Ans:B
It is an acid-base reaction in which \(H_2O\) donates \(H^+\) to \(NH_3\), forming \(NH_4^+\) and \(OH^−\) ions in solution.
Question
\(2Mg(s)+SiCl_4(l)→2MgCl_2(s)+Si(s)\)
Which of the following statements about the reaction represented above is correct?
A It is an oxidation-reduction reaction, and Mg is oxidized.
B It is an oxidation-reduction reaction, and electrons are transferred from \(SiCl_4\) to Mg
C It is an oxidation-reduction reaction, and the oxidation number of Cl changes from +4 to +2 .
D It is not an oxidation-reduction reaction because none of the oxidation numbers change.
▶️Answer/Explanation
Ans:A
In an oxidation-reduction reaction electrons are transferred from one species to another. Mg is oxidized; it loses electrons, as indicated by its oxidation number changing from 0 to +2.
Question
__________ electrons appear in the following half-reaction when it is balanced.
\(S_4O_6^{2-} \rightarrow 2S_2O_3^{2-}\)
A) 4 B) 1 C) 2 D) 6 E) 3
▶️Answer/Explanation
Ans: C
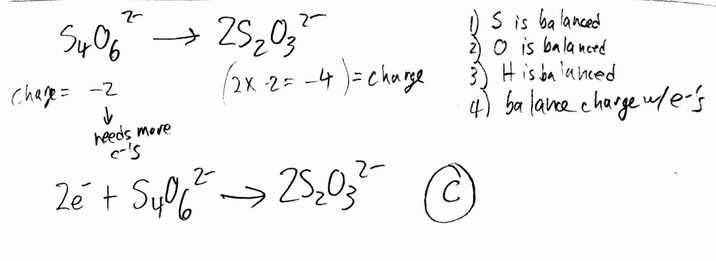
Question
Which of the following best describes the role of the spark from the spark plug in an automobile engine?
(A) The spark decreases the energy of activation for the slow step.
(B) The spark increases the concentration of the volatile reactant.
(C) The spark supplies some of the energy of activation forthe combustion reaction.
(D) The spark provides a more favorable activated complex for the combustion reaction.
(E) The spark provides the heat of vaporization for the volatile hydrocarbon.
▶️Answer/Explanation
Ans: C