Question
Answer the following questions relating to the chemistry of the halogens.
(a) The molecular formulas of diatomic bromine, chlorine, fluorine, and iodine are written below. Circle the formula of the molecule that has the longest bond length. Justify your choice in terms of atomic structure.
Br2 Cl2 F2 I2
A chemistry teacher wants to prepare Br2 . The teacher has access to the following three reagents: NaBr(aq), Cl2(g), and I2(s).
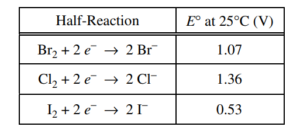
(b) Using the data in the table above, write the balanced equation for the thermodynamically favorable reaction that will produce Br2 when the teacher combines two of the reagents. Justify that the reaction is thermodynamically favorable by calculating the value of E° for the reaction.
Br2 and Cl2 can react to form the compound BrCl.
(c) The boiling point of Br2 is 332 K, whereas the boiling point of BrCl is 278 K. Explain this difference in boiling point in terms of all the intermolecular forces present between molecules of each substance.
The compound BrCl can decompose into Br2 and Cl2 , as represented by the balanced chemical equation below.
2 BrCl(g) ⇔ Br2(g) + Cl2(g) ΔH° = 1.6 kJ/molrxn
A 0.100 mole sample of pure BrCl(g) is placed in a previously evacuated, rigid 2.00 L container at 298 K. Eventually the system reaches equilibrium according to the equation above.
(d) Calculate the pressure in the container before equilibrium is established.
(e) Write the expression for the equilibrium constant, Keq , for the decomposition of BrCl.
After the system has reached equilibrium, 42 percent of the original BrCl sample has decomposed.
(f) Determine the value of Keq for the decomposition reaction of BrCl at 298 K.
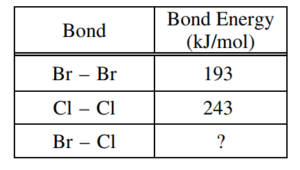
(g) Calculate the bond energy of the Br-Cl bond, in kJ/mol, using ΔH° for the reaction (1.6 kJ/molrxn) and the information in the following table.
▶️Answer/Explanation
Ans:
(a)
| I2 has the longest bond length because the radius of he I atom is greater than the radii of the other halogen atoms. Thus, the distance between the nuclei of atoms in I2 is greater than it is in smaller halogens. |
(b)
2 Br– + Cl → Br2 + 2 Cl– E0 = E0 (reduced species) – E0 (oxidized species) Because E0 for the reaction has a positive value, the reaction is thermodynamically favorable. |
(c)
| The only intermolecular attractions in Br2(l) are London forces, while those in BrCl(l) include both London forces and dipole-dipole forces. However, due to the greater polarizability of the electron cloud of Br2 compared to that of BrCl, the London forces in Br2(l) are stronger than the combined intermolecular forces in BrCl(l). Thus the boiling point of Br2(l) is greater than that of BrCl(l). |
(d)
| \(P=\frac{nRT}{V}=\frac{(0.100 mol)(0.08206 L atm mol^{-1}K^{-1})(298 K)}{2.00 L}=1.22 atm\) |
(e)
| \(K_{eq}=\frac{[Br_{2}][Cl_{2}]}{[BrCl]^{2}} \) or \(K_{eq}=\frac{P_{Br_{2}}P_{Cl_{2}}}{(P_{BrCl})^{2}}\) |
(f)
2 BrCl(g) ⇔ Br2(g) + Cl2(g)
PBrCl decomposed = (0.42) (1.22 atm) = 0.51 atm \(K_{eq}=\frac{(0.26)(0.26)}{(0.71)^{2}}=0.13\) Note: The solution is in terms of pressures. Solutions in terms of molar concentrations also earn full credit. |
(g)
ΔH0 = ∑ (bond energies) broken – ∑ (bond energies) formed 1.6 kJ/mol = 2(Br-Cl bond energy) – 193 kJ/mol + 243 kJ/mol) (436 + 1.6) kJ/mol = 2 (Br – Cl bond energy) Br – Cl bond energy = 219 kJ/mol |
Question
NaHCO3(s) + HC2H3O2(aq) → NaC2H3O2(aq) + H2O(l) + CO2(g)
A student designs an experiment to study the reaction between NaHCO3 and HC2H3O2 . The reaction is represented by the equation above. The student places 2.24 g of NaHCO3 in a flask and adds 60.0 mL of 0.875 M HC2H3O2 . The student observes the formation of bubbles and that the flask gets cooler as the reaction proceeds.
(a) Identify the reaction represented above as an acid-base reaction, precipitation reaction, or redox reaction. Justify your answer.
(b) Based on the information above, identify the limiting reactant. Justify your answer with calculations.
(c) The student observes that the bubbling is rapid at the beginning of the reaction and gradually slows as the reaction continues. Explain this change in the reaction rate in terms of the collisions between reactant particles.
(d) In thermodynamic terms, a reaction can be driven by enthalpy, entropy, or both.
(i) Considering that the flask gets cooler as the reaction proceeds, what drives the chemical reaction between NaHCO3(s) and HC2H3O2(aq) ? Answer by drawing a circle around one of the choices below.
Enthalpy only Entropy only Both enthalpy and entropy
(ii) Justify your selection in part (d)(i) in terms of ΔG°.
(e) The HCO3− ion has three carbon-to-oxygen bonds. Two of the carbon-to-oxygen bonds have the same length and the third carbon-to-oxygen bond is longer than the other two. The hydrogen atom is bonded to one of the oxygen atoms. In the box below, draw a Lewis electron-dot diagram (or diagrams) for the HCO3− ion that is (are) consistent with the given information.

(f) A student prepares a solution containing equimolar amounts of HC2H3O2 and NaC2H3O2 . The pH of the solution is measured to be 4.7. The student adds two drops of 3.0 M HNO3(aq) and stirs the sample, observing that the pH remains at 4.7. Write a balanced, net-ionic equation for the reaction between HNO3(aq) and the chemical species in the sample that is responsible for the pH remaining at 4.7.
▶️Answer/Explanation
Ans:
(a)
| It is an acid-base reaction. The weak acid HC2H3O2 reacts with the weak base HCO3– with HC2H3O2 donating a proton. OR It is an acid-base reaction. No solid precipitates, so it is not a precipitation reaction. None of the oxidation numbers change, so it is not a redox reaction. |
(b)
\(2.24 g NaHCO_{3}\times \frac{1 mol NaHCO_{3}}{84.0 g}= 0.0267 mol NaHCO_{3}\) \(60.0 mL\times \frac{0.875 mol HC_{2}H_{3}O_{2}}{1000 mL}= 0.0525 mol HC_{2}H_{3}O_{2}\) The NaHCO3(s) and HC2H3O2 (aq) react in a 1:1 ratio, so the limiting reactant is NaHCO3(s). |
(c)
| As the reaction proceeds, both reactants are consumed and their concentrations decrease. Collisions between reactant particles become less likely as their concentrations decrease, thus the reaction rate slows. |
(d) (i)
| Entropy only should be circled. |
(ii)
ΔG0 = ΔH0 – TΔS0 Reactions are thermodynamically favorable when ΔG0 is negative. Since the reaction is endothermic (the flask cooler, ΔH0 is positive), the reaction is not driven by enthalpy, because enthalpy does not help make ΔG0 negative. Because there are no gases in the reactants and one of the products is a gas, ΔS0 must be positive, which helps make ΔG0 negative. |
(e)

| See diagram above. |
(f)
| C2H3O2– + H3O+ → HC2H3O2 + H2O OR C2H3O2– + H+ → HC2H3O2 |
