Question
\(2 NO(g) + Br_2(g) \rightarrow 2 NOBr(g)\)
A rate study of the reaction represented above was conducted at 25°C. The data that were obtained are shown in
the table below.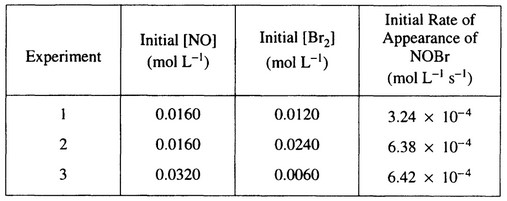
(a) Calculate the initial rate of disappearance of \(Br_2(g)\) in experiment
(b) Determine the order of the reaction with respect to each reactant, \(Br_2(g)\) and NO(g). In
explain your reasoning.
(c) For the reaction,
(i) write the rate law that is consistent with the data, and
(ii) calculate the value of the specific rate constant, k, and specify units.
(d) The following mechanism was proposed for the reaction:
\(Br_2(g) + NO(g) \rightarrow NOBr_2(g)\) slow
\(NOBr_2(g) + NO(g) \rightarrow 2 NOBr(g)\) fast
Is this mechanism consistent with the given experimental observations? Justifv vour answer.
▶️Answer/Explanation
Answer:
(a) Rate of \(Br_2(g)\) loss occurs at 1/2 the rate of NOBr(g) formation.
Rate of \(Br_2(g)\) loss = \(\frac{3.24 \times 10^{-4} M}{2 sec}=1.62 \times 10^{-4} M sec^{-1} (or mol L^{-1 }sec^{-1})\)
(b) Comparing experiments 1 and 2, [NO] remains constant, \([Br]_2\) doubles, and rate doubles;
therefore, rate ∝ \([Br_2]^1 \Rightarrow \) reaction is first-order with respect to \([Br_2]\).
\(\frac{6.38 \times 10^{-4}}{6.42 \times 10^{-4}} ≅ 1 = \frac{k[NO]^x[Br_2]}{k[NO]^x[Br_2]}=\frac{k[0.0160]^x[0.0240]}{k[0.0320]^x[0.0060]}=(\frac{1}{2})^x4 = 1 \Rightarrow\)
\(\frac{1}{4}=(\frac{1}{2})^x \Rightarrow x = 2 \Rightarrow \) reaction is second-order with respect to [NO]
(c) (i) Rate = \(k[NO]^2[Br_2]\)
(ii) \(k=\frac{Rate}{[NO]^2[Br_2]}=\frac{3.24 \times 10^{-4} M sec^{-1}}{(0.0160)^2(0.0120)M^3}=105 M^{-2} sec^{-1}~ (or~ 105 L^2 mol^{-2} sec^{-1})\)
(Using rate of \(Br_2(g)\) \(loss = 1.62 \times 10^{-4} M sec^{-1} \Rightarrow k = 52.7 M^{-2} sec^{-1}\) is also correct).
(d) No, it is not consistent with the given experimental observations.
This mechanism gives a reaction that is first-order in [NO], and first-order in \([Br_2]\), as those are the two reactants in the rate-determining step. Kinetic data show the reaction is second-order in [NO] (and first-order in \([Br_2])\), so this cannot be the mechanism.
Question
2 NO(g) + 2 H2(g) + N2(g) + 2 H2O(g)
Experiments were conducted to study the rate of the reaction represented by the equation above. Initial concentrations and rates of reaction are given in the table below.
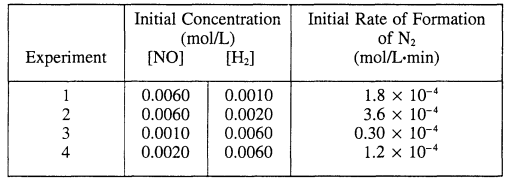
(a (i) Determine the order for each of the reactants, NO and H2, from the data given and show your reasoning.
(ii) Write the overall rate law for the reaction.
(b) Calculate the value of the rate constant, k, for the reaction. Include units.
(c) For experiment 2, calculate the concentration of NO remaining when exactly one-half of the original amount of H2 had been consumed.
(d) The following sequence of elementary steps is a proposed mechanism for the reaction.
I. NO + NO⇔N2O2
II. N2O2 + H2 →H2O + N20
III. N2O + H2 → N2 + H2O
Based on the data presented, which of the above is the rate-determining step? Show that the mechanism is consistent with
(i) the observed rate law for the reaction, and
(ii) the overall stoichiometry of the reaction.
▶️Answer/Explanation
Ans:
(a) (i) From exps. 1 and 2: Doubling [H2] while keeping [NO] constant doubles the rate, therefore the reaction is first order in [H2].
From exps. 3 and 4: Doubling [NO] while keeping [H2 ] constant quadruples the rate, therefore the reaction is second order in [NO].
(ii) Rate = k[H2] [NO]2
Note: full credit earned for (ii) as long as rate expression is consistent with orders in (i).
(b) \(k = \frac{Rate}{[H_{2}][NO]^{2}}\)
From exp. 1: \(k = \frac{1.8\times 10^{-4}M/min}{(1.0\times 10^{-3}M)(6.0\times 10^{-3}M)^{2}}\)
= 5.0 × 103 M-2 min-1
Note: same result from initial rate data from all 4 experiments.
(c) Stoichiometry: NO:H2 is 1:1 When 0.0010 mole of H2 had reacted, it must have reacted with 0.0010 mole of NO; thus [NO] remaining = 0.0060 – 0.0010 – 0.0050 M.
(d) (i) For I : \(K_{eq}=\frac{[N_{2}O_{2}]}{[NO]^{2}}\)
For II: Rate = k[H2] [N2O2]
[N2O2] = Keq[NO]2
Rate = k'[H2][NO]2
Note: there must be some clear algebraic manipulation showing that [N2O2] is proportional (NOT equal) to [NO]2.
Step II is the rate-determining step.
(ii) I : NO + NO N2O2
II : N2O2 + H2 → H2O + N2O
II : N2O + H2 → N2 + H2O
I + II + III : 2 NO + 2H2 → N2 + 2 H2O