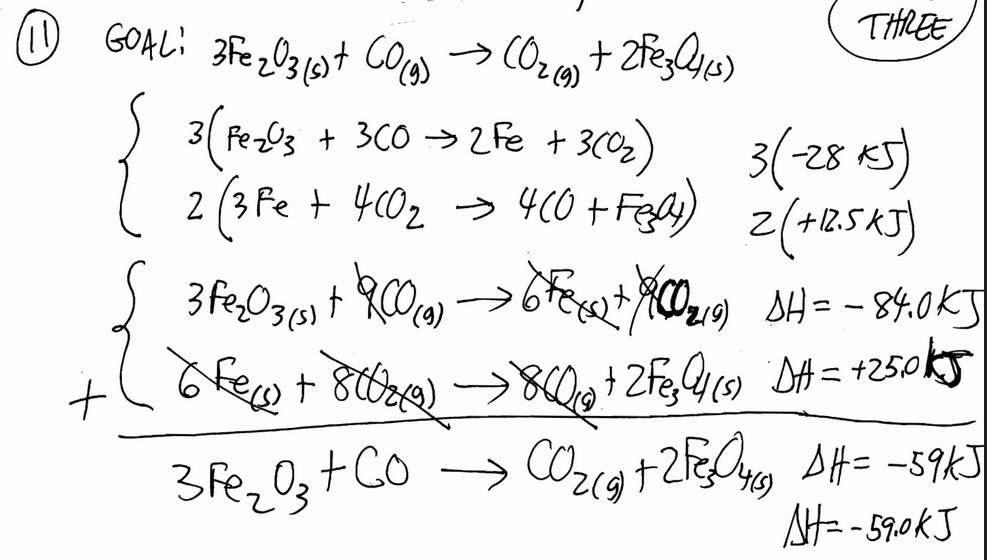Questions
Which of the following is a list of the minimum amount of data needed for determining the molar enthalpy of solution of KCl(s) in pure \(H_2O\)(l) ? (Assume that the KCl(aq) has the same specific heat capacity as pure water and that the initial temperatures of the KCl(s) and the water are the same.)
(A) Mass of KCl(s), initial temperature of the water, and final temperature of the solution
(B) Mass of \(H_2\)O, initial temperature of the water, and final temperature of the solution
(C) Mass of KCl(s), mass of H2O, initial temperature of the water, and final temperature of the solution
(D) Mass of KCl(s), mass of\( H_2\)O, initial temperature of the water, final temperature of the solution, and atmospheric pressure
▶️Answer/Explanation
Ans: C
To determine the molar enthalpy of solution (AHsoln) of KCl(s) in pure H2O(1), we need to know the heat absorbed or released during the dissolution process. This heat can be calculated using the specific heat
capacity, the mass of the solution, and the temperature change.
Given the assumptions that the KCl(aq) has the same specific heat capacity as pure water and that the initial temperatures of KCI(s) and water are the same, the minimum amount of data needed is:
(C) Mass of KCI(s), mass of H2O, initial temperature of the water, and final temperature of the solution.
Here’s why each piece of data is required:
1. Mass of KCI(s): Needed to determine the number of moles of KCl dissolved and relate the heat change to the molar enthalpy of solution.
2. Mass of H20: Needed to calculate the total mass of the solution and, along with the temperature change, determine the heat absorbed or released during the dissolution process.
3. Initial temperature of the water: Needed to calculate the temperature change when combined with the final temperature of the solution.
4. Final temperature of the solution: Needed to calculate the temperature change when combined with the initial temperature of the water.
Atmospheric pressure (option D) is not necessary, as the enthalpy of solution is typically measured at constant pressure, and the atmospheric pressure does not affect the calculation.
The other options (A and B) are missing either the mass of KCI(s) or the mass of H20, which are essential data points for determining the molar enthalpy of solution.
Questions
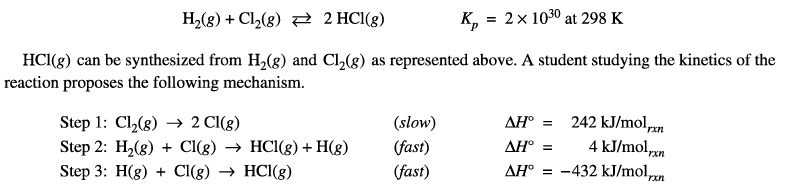
What is the value of the enthalpy change per mole of HCl(g) produced?
(A) -93 kJ
(B) -121 kJ
(C) -186 kJ
(D) -242 kJ
▶️Answer/Explanation
Ans: A
To find the value of the enthalpy change per mole of \( HCl(g) \) produced, we need to divide the overall standard enthalpy change (\( \Delta H^\circ \)) by the stoichiometric coefficient of \( HCl(g) \) in the balanced equation.
The balanced equation is:
\[ H_2(g) + Cl_2(g) \rightleftharpoons 2 HCl(g) \]
The overall standard enthalpy change (\( \Delta H^\circ \)) was calculated to be \( -186 \, \text{kJ/mol} \).
The stoichiometric coefficient of \( HCl(g) \) is 2.
Therefore, the enthalpy change per mole of \( HCl(g) \) produced is:
\[ \Delta H^\circ \text{ per mole of } HCl(g) = \frac{{-186 \, \text{kJ/mol}}}{{2}} \]
\[ \Delta H^\circ \text{ per mole of } HCl(g) = -93 \, \text{kJ/mol} \]
Hence, the correct answer is (A) -93 kJ.
Questions

The process of dissolution of NaCl(s) in \(H_{2}O_{(l)}\) is represented in the diagram above. Which of the following summarizes the signs of \( \bigtriangleup H^{\circ }\) and \( \bigtriangleup S^{\circ }\) for each part of the dissolution process?

▶️Answer/Explanation
Ans: A
Breaking solvent-solvent interactions:
In the dissolution process, the water molecules (solvent) initially have strong hydrogen bonding interactions with each other. When a solute like NaCl is added, these solvent-solvent interactions need to be broken to accommodate the solute particles. Breaking these strong intermolecular forces is an endothermic process (\(\Delta H^\circ > 0\)), as energy needs to be absorbed to overcome the attractive forces. Additionally, breaking the ordered structure of water molecules leads to an increase in disorder or entropy (\(\Delta S^\circ > 0\)).
Breaking solute-solute interactions:
In the solid state, NaCl ions are held together by strong ionic bonds. When NaCl dissolves, these ionic bonds need to be broken, which requires energy input, making it an endothermic process (\(\Delta H^\circ > 0\)). Moreover, separating the ions from their ordered crystal lattice increases the disorder or entropy (\(\Delta S^\circ > 0\)).
Forming solute-solvent interactions:
After the solvent-solvent and solute-solute interactions are broken, the solute ions (\(Na^+\) and \(Cl^-\)) interact with the water molecules (solvent) to form new solute-solvent interactions. These new interactions involve ion-dipole attractions and hydrogen bonding, which are exothermic processes (\(\Delta H^\circ < 0\)), releasing energy. However, the formation of these interactions results in a more ordered system, decreasing the entropy (\(\Delta S^\circ < 0\)).
Therefore, option (A) correctly summarizes the signs of \(\Delta H^\circ\) and \(\Delta S^\circ\) for each part of the dissolution process, where both breaking solvent-solvent and solute-solute interactions are endothermic (\(\Delta H^\circ > 0\)) and increase entropy (\(\Delta S^\circ > 0\)), while forming solute-solvent interactions is exothermic (\(\Delta H^\circ < 0\)) and decreases entropy (\(\Delta S^\circ < 0\)).
Question
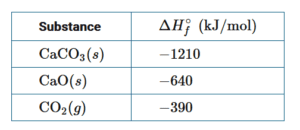
\(CaCO_3\) decomposes according to the balanced equation \(CaCO_3(s)→CaO(s)+CO_2(g)\).Based on the standard enthalpies of formation provided in the table above, what is the approximate enthalpy change of the reaction?
A −180kJ
B +180kJ
C −1460kJ
D +1460kJ
▶️Answer/Explanation
Ans:B
The enthalpy change for a chemical reaction can be estimated using \(\Delta H^{0}_{rxn}=\sum \Delta H^{0}_{f}{,products}-\sum \Delta H^{0}_{f}{ , reactants}\) Based on the reaction and the values provided,
\([ΔH_{rxn}]=[(1mole)\Delta H_{f}^0(CAO)+(1mole)\Delta H_{f}^0(CO_2)]-[(1mole)\Delta H_{f}^0(CaCO_3)]\)
\([-640kJ+(-390kJ)-(-1210kJ)]\rightarrow +180kJ\)
Question
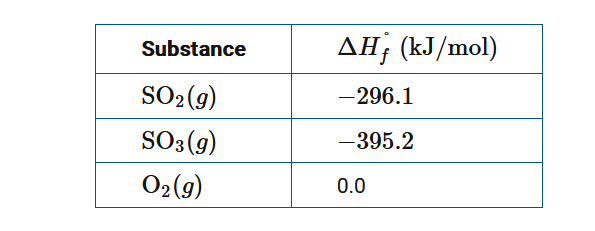
Based on the information above, what is ΔH° for the reaction \(SO_2(g)+\frac{1}{2}O_2(g)→SO_3(g)\) ?
A−591.3kJ/molrxn
B −99.1kJ/molrxn
C +99.1kJ/molrxn
D +591.3kJ/molrxn
▶️Answer/Explanation
Ans:B
The standard enthalpy of reaction (ΔH°) is equal to \(\sum \Delta H^0_f,products-\sum \Delta H^0_f,reactants\) ; thus,ΔH°\(=(-359.2kJ/mol)-(-296.1 kJ/mol)=-99.1kJ/mol_{rxn}\).
Question
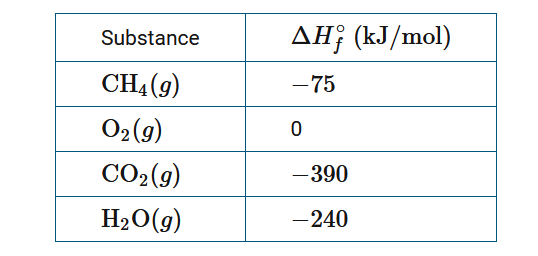
Natural gas consists primarily of \(CH_4\) , which is combusted according to the following chemical equation.
\(CH_4(g)+2O_2(g)→CO_2(g)+2H_2O(g)\)
Based on the standard enthalpies of formation in the table above, which of the following expressions give the approximate enthalpy change for the reaction \(\Delta H^0_{rxn\)?
A \(\Delta H^0_{rxn}=[(−75kJ/mol)−[(−390kJ/mol)+2(−240kJ/mol)]\)
B \(\Delta H^0_{rxn}=[(−75kJ/mol)−[(−390kJ/mol)+(−240kJ/mol)]\)
C \(\Delta H^0_{rxn}=[(−390kJ/mol)+2(−240kJ/mol)]−(−75kJ/mol)]\)
D \(\Delta H^0_{rxn}=[(−390kJ/mol)+(−240kJ/mol)]−(−75kJ/mol)]\)
▶️Answer/Explanation
Ans:C
The enthalpy change for a reaction can be estimated from \(\sum \Delta H^0_f,products-\sum \Delta H^0_f,reactants\) . Using the values given, \(\Delta H^0_{rxn}=[(−390kJ/mol)+2(−240kJ/mol)]−(−75kJ/mol)\).
Question

Natural gas consists primarily of \(CH_4\) , which is combusted according to the following chemical equation.
\(CH_4(g)+2O_2(g)→CO_2(g)+2H_2O(g)\)
Based on the standard enthalpies of formation in the table above, which of the following expressions give the approximate enthalpy change for the reaction \(\Delta H^0_{rxn\)?
A \(\Delta H^0_{rxn}=[(−75kJ/mol)−[(−390kJ/mol)+2(−240kJ/mol)]\)
B \(\Delta H^0_{rxn}=[(−75kJ/mol)−[(−390kJ/mol)+(−240kJ/mol)]\)
C \(\Delta H^0_{rxn}=[(−390kJ/mol)+2(−240kJ/mol)]−(−75kJ/mol)]\)
D \(\Delta H^0_{rxn}=[(−390kJ/mol)+(−240kJ/mol)]−(−75kJ/mol)]\)
▶️Answer/Explanation
Ans:C
The enthalpy change for a reaction can be estimated from \(\sum \Delta H^0_f,products-\sum \Delta H^0_f,reactants\) . Using the values given, \(\Delta H^0_{rxn}=[(−390kJ/mol)+2(−240kJ/mol)]−(−75kJ/mol)\).
Question
Refer to the following graph, which shows the heating curve for methane, \(CH_4\)
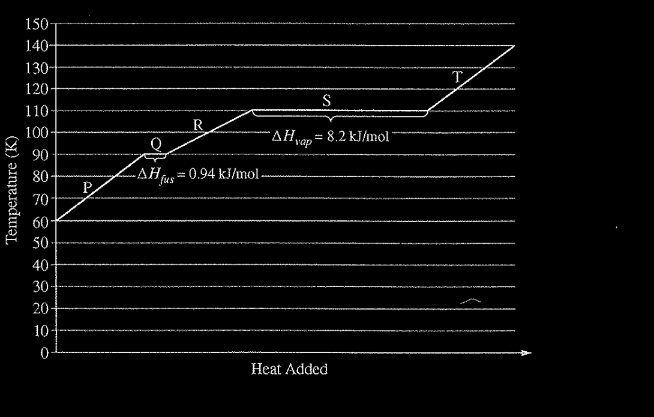
The enthalpy of vaporization of water is 40.7 kJ/mol. Which of the following best explains why the enthalpy of vaporization of methane is less than that of water?
(A) Methane does not exhibit hydrogen bonding, but water does.
(B) Methane has weaker dispersion forces.
(C) Methane has a smaller molar mass.
(D) Methane has a much lower density.
▶️Answer/Explanation
Ans:A
Question
Given the following reactions
\(Fe_2O_3 (s) + 3CO (s) \rightarrow 2Fe (s) + 3CO_2 (g)\) ∆H = -28.0 kJ
\(3Fe (s) + 4CO_2 (s) \rightarrow 4CO (g) + Fe_3O_4 (s)\) ∆H = +12.5 kJ
the enthalpy of the reaction of \(Fe_2O_3\) with CO
\(3Fe_2O_3 (s) + CO (g) \rightarrow CO_2 (g) + 2Fe_3O_4 (s)\) is ________ kJ.
A) 40.5 B) +109 C) -15.5 D) -109 E) -59.0
▶️Answer/Explanation
Ans: E