Question
Which of the following Lewis electron-dot diagrams represents the molecule that is the most polar?

▶️Answer/Explanation
Ans:B
To determine which molecule is the most polar, we need to consider the electronegativities of the atoms involved and the molecular geometry.
1. \(ClF\):
Chlorine (\(Cl\)) is more electronegative than fluorine (\(F\)).
The molecule has a bent geometry with one lone pair on chlorine and two bonding pairs.
This lone pair contributes to the overall polarity of the molecule.
The polarity of \(ClF\) is significant but less than other molecules in the list.
2. \(BRF\):
Bromine (\(Br\)) is more electronegative than fluorine (\(F\)).
The molecule has a linear geometry with three bonding pairs.
Due to the higher electronegativity of bromine compared to boron (\(B\)), there is a significant dipole moment.
This molecule is highly polar.
3. \(CS_2\):
Carbon (\(C\)) is less electronegative than sulfur (\(S\)).
The molecule has a linear geometry with two sulfur atoms and a carbon atom.
Since the sulfur atoms are at both ends of the molecule and have similar electronegativities, the dipole moments cancel out, resulting in a nonpolar molecule.
4. \(BF_3\):
Boron (\(B\)) is less electronegative than fluorine (\(F\)).
The molecule has a trigonal planar geometry with three bonding pairs.
The dipole moments of the three \(\text{B-F}\) bonds cancel out due to the symmetrical arrangement of the fluorine atoms around boron.
This molecule is nonpolar.
So, the most polar molecule among the given options is \(BRF\).
Question
Based on the octet rule, magnesium most likely forms a __________ ion.
A) Mg- B) \(Mg^{6+}\) C) \(Mg^{2+}\) D) \(Mg^{6-}\) E) \(Mg^{2-}\)
▶️Answer/Explanation
Ans: C
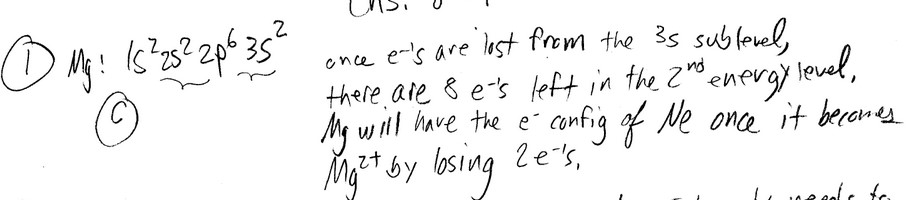
Question
Based on the octet rule, phosphorus most likely forms a __________ ion.
A) \(P^{5-}\) B) \(P^{5+}\) C) \(P^+\) D) \(P^{3-}\) E) \(P^{3+}\)
▶️Answer/Explanation
Ans: D
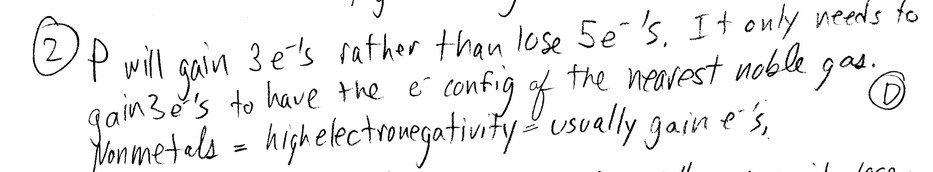
Question
The Lewis structure of \(AsH_3\) shows __________ nonbonding electron pair(s) on As.
A) 0
B) 1
C) 2
D) 3
E) This cannot be determined from the data given.
▶️Answer/Explanation
Ans: B
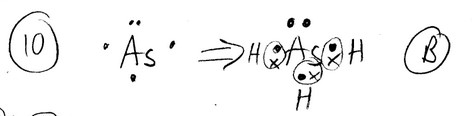
Question
The Lewis structure of HCN (H bonded to C) shows that __________ has __________ nonbonding electron
pairs.
A) C, 1 B) N, 2 C) C, 2 D) N, 1 E) H, 1
▶️Answer/Explanation
Ans: D
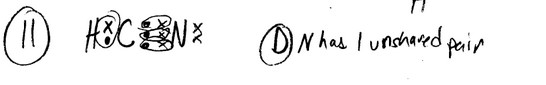
Question
The Lewis structure of \(N_2H_2\) shows __________.
A) each nitrogen has one nonbinding electron pair
B) each hydrogen has one nonbonding electron pair
C) each nitrogen has two nonbinding electron pairs
D) a nitrogen-nitrogen single bond
E) a nitrogen-nitrogen triple bond
▶️Answer/Explanation
Ans: A
