Question
Answer the following questions using principles of chemical bonding and molecular structure.
(a) Consider the carbon dioxide molecule, \(CO_2\) , and the carbonate ion, \(CO_3^{2-}\).
(i) Draw the complete Lewis electron-dot structure for each species.
(ii) Account for the fact that the carbon-oxygen bond length in \(CO_3^{2-}\) is greater than the carbon-oxygen bond length in CO, .
(b) Consider the molecules \(CF_4\) and \(SF_4\) .
(i) Draw the complete Lewis electron-dot structure for each molecule.
(ii) In terms of molecular geometry, account for the fact that the \(CF_4\) molecule is nonpolar, whereas the
\(SF_4\) molecule is polar.
▶️Answer/Explanation
Answer:
(a) (i) 
- One point earned for each Lewis electron-dot structure
- Indication of lone pairs of electrons are required on each structure
- Resonance forms of \(CO_3^{2-}\) are not required
(ii) In \(CO_2\), the C-O interactions are double bonds, OR, in \(CO_3^{2-}\) the C-O interactions are resonance forms (or figures below.)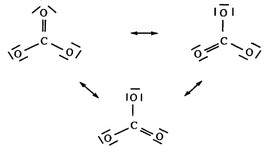
The carbon-oxygen bond length is greater in the resonance forms than in the double bonds.
- \(1^{st}\)point earned for indicating double bonds are present in \(CO_2\) OR resonance occurs in \(CO_3^{2-}\)
- \(2^{nd}\) point earned for BOTH of the above AND indicating the relative lengths of the bond types
(b) (i) 
- One point earned for each Lewis electron-dot structure
- Lone pairs of electrons are required on each structure
(ii) \(CF_4\) has a tetrahedral geometry, so the bond dipoles cancel, leading to a nonpolar molecule.
With five pairs of electrons around the central S atom, \(SF_4\) exhibits a trigonal bipyramidal electronic geometry, with the line pair of electrons. In this configuration, the bond dipoles do not cancel, and the molecules is polar.

- One point earnedfor each molecule for proper geometry and explanation
Question
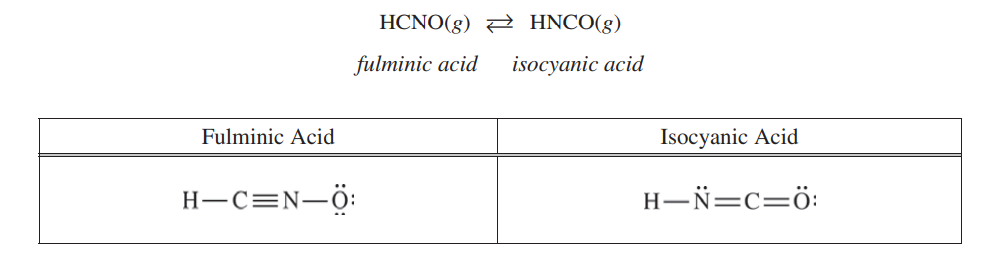
Answer the following questions about the isomers fulminic acid and isocyanic acid. Two possible Lewis electron-dot diagrams for fulminic acid, HCNO, are shown below.
![]()
(a) Explain why the diagram on the left is the better representation for the bonding in fulminic acid. Justify your choice based on formal charges.
Fulminic acid can convert to isocyanic acid according to the equation below.
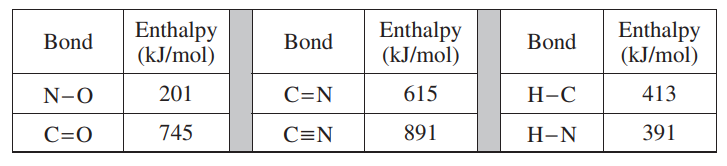
(b) Using the Lewis electron-dot diagrams of fulminic acid and isocyanic acid shown in the boxes above and the table of average bond enthalpies below, determine the value of ‘H° for the reaction of HCNO(g) to form HNCO(g).
(c) A student claims that ΔS° for the reaction is close to zero. Explain why the student’s claim is accurate.
(d) Which species, fulminic acid (HCNO) or isocyanic acid (HNCO), is present in higher concentration at equilibrium at 298 K? Justify your answer in terms of thermodynamic favorability and the equilibrium constant.
The ammonium salt of isocyanic acid is a product of the decomposition of urea, CO(NH2)2 , represented below.
CO(NH2)2(aq) R NH4+(aq) + OCN−(aq)
A student studying the decomposition reaction runs the reaction at 90°C. The student collects data on the concentration of urea as a function of time, as shown by the data table and the graph below.
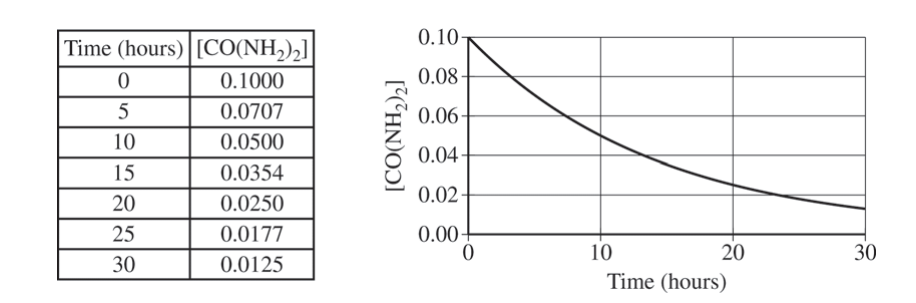
(e) The student proposes that the rate law is rate = k[CO(NH2)2].
(i) Explain how the data support the student’s proposed rate law.
(ii) Using the proposed rate law and the student’s results, determine the value of the rate constant, k. Include units with your answer.
(f) The student learns that the decomposition reaction was run in a solution with a pH of 13. Briefly describe an experiment, including the initial conditions that you would change and the data you would gather, to determine whether the rate of the reaction depends on the concentration of OH−(aq).
▶️Answer/Explanation
Ans:
(a)
In the diagram on the left, the C atom has a formal charge of zero and the O atom has a formal charge of -1. In the diagram on the right, the C atom has a formal charge of -1 and the O atom has a formal charge of zero. The diagram on the left is the better representation because it puts the negative formal charge on oxygen, which is more electronegative than carbon. |
(b)
ΔH0 = ∑ (enthalpies of bonds broken) – ∑(enthalpies – of bonds formed) |
(c)
| The change from fulminic acid to isocyanic acid is a rearrangement of atoms with no change in phas or number of molecules. |
(d)
Isocyanic acid (HNCO) will be present in higher concentration. ΔG0 is essentially equal to ΔH0 because ΔS0 is essentially zero, so ΔG0 ≈ -246 kJ/molrxn, indicating the forward reaction is thermodynamically favorable. |
(e) (i)
| From inspecting the data table or the graph, it is evident that the decomposition reaction has a constant half-life, which indicates that the reaction is a first-order reaction. |
(ii)
Since the reaction is first order, \(k = \frac{0.693}{t_{1/2}}=\frac{0.693}{10.h}=0.069 h^{-1}\) OR \(k = \frac{1n[A]_{0}-1n[A]_{t}}{t}=\frac{1n (0.1000)-1n(0.0500)}{10.h}=0.069 h^{-1}\) |
(f)
| Perform the experiment at a different concentration of OH–(aq) and measure how the concentration of C(NH2)2 changes over time. (Other variable, such as temperature, should be held constant.) |
