Question

Equal volumes of solutions in two different vessels are represented above. If the solution represented in vessel 1 is KCl(aq), then them solution represented in vessel 2 could be an aqueous solution of
(A) KCl with the same molarity as the solution in vessel 1
(B) KCl with twice the molarity of the solution in vessel 1
(C) \( CaCl_{2}\) with the same molarity as the solution in vessel 1
(D) \(CaCl_{2}\) with twice the molarity of the solution in vessel 1
▶️Answer/Explanation
Ans:D
Based on the image, Vessel 1 contains an equal number of positive and negative ions, indicating a 1:1 electrolyte like KCl. In Vessel 2, there are more positive ions than negative ions, suggesting a higher ratio of positive to negative ions compared to Vessel 1.
Therefore, the correct option is (D) CaCl₂ with twice the molarity of the solution in Vessel 1.
The higher ratio of positive to negative ions in Vessel 2 is consistent with the presence of CaCl₂, which has a 2:1 ratio of positive to negative ions (Ca²⁺ and 2Cl⁻). Additionally, the higher number of ions overall in Vessel 2 compared to Vessel 1 suggests a higher molarity (twice the molarity of Vessel 1’s KCl solution).
Question
What volume of a 0.100M HCl stock solution should be used to prepare 250.00mL of 0.0250M HCl?
▶️Answer/Explanation
Ans: C
When using a stock solution to prepare a solution of lower concentration, only solvent is added and the number of moles of solute remain constant. In these situations, the volume of stock solution can be obtained by rearranging the equation: \(M_1V_1=M_2V_2\). Therefore, \(V_1=\frac{M_2V_2}{M_1}\)=0.0250M×250.00mL0.100M=62.5mL. Note that when working with ratios, the units of volume will be the same as those of the volume given.
Question
A 500.mL aqueous solution of \(Na_3PO_4\) (molarmass=164g/mol) was prepared using 82g of the solute. What is the molarity of \(Na_3PO_4\) in the resulting solution?
A 0.0010M
B 0.16M
C 0.25M
D 1.0M
▶️Answer/Explanation
Ans: D
To calculate the molarity of a solution, the number of moles of solute is divided by the volume of the solution in liters. Therefore, \( \frac{82/164}{500/1000}=1.0\) M
Question
\(C_3H_8(g) + 4 Cl_2(g) → C_3H_4Cl(g) + 4 HCl(g)\)
A 6.0 mol sample of\( C_{3}H_8(g)\) and a 20. mol sample of \(Cl_{2}\)(g) are placed in a previously evacuated vessel, where they react according to the equation above. After one of the reactants has been totally consumed, how many moles of HCl(g) have been produced?
(A) 4.0 mo
(B) 8.0 mol
(C) 20. mol
(D) 24 mol
▶️Answer/Explanation
Ans:C
Question
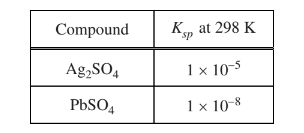
A 1.0 L solution of \(AgNO_{3}\)(aq) and Pb\((NO_{3})_{2}\)(aq) has a \(Ag^{+}\) concentration of 0.020 M and a\( Pb^{2+}\) concentration of 0.0010 M. A 0.0010 mol sample of \(K_{2}SO_{4}\)(s) is added to the solution. Based on the information in the table above, which of the following will occur? (Assume that the volume change of the solution is negligible.)
(A) No precipitate will form.
(B) Only \(Ag_{2}SO_{4}\)(s) will precipitate.
(C) Only \(PbSO_{4}\)(s) will precipitate.
(D) Both \(Ag_{2}SO_{4}\)(s) and \(PbSO_{4}\)(s) will precipitate.
▶️Answer/Explanation
Ans:C
Question
\(Fe(s) + 2 HCl(aq) \rightarrow FeCl_{2}(aq) + H_{2}(g)\)
When a student adds 30.0 mL of 1.00 M HCl to 0.56 g of powdered Fe, a reaction occurs according to the equation above. When the
reaction is complete at 273 K and 1.0 atm, which of the following is true?
(A) HCl is in excess, and 0.100 mol of HCl remains unreacted.
(B) HCl is in excess, and 0.020 mol of HCl remains unreacted.
(C) 0.015 mol of \(FeCl_{2}\) has been produced.
(D) 0.22 L of\( H_{2} \)has been produced.
▶️Answer/Explanation
Ans:D
Question.
\(Fe^{3+}{aq}+KSCN(s)\rightarrow FeSCN^{2+}(aq)+K(aq)\)
. To determine the moles of \(Fe^{3+}\)(aq) in a 100. mL sample of an unknown solution, excess KSCN(s) is added to convert all the Fe\(^{3+}\)(aq) into the dark red species\( FeSCN^{2+}\)(aq), as represented by the equation above. The absorbance of\( FeSCN^{2+}\)(aq) at different concentrations is shown in the graph below.
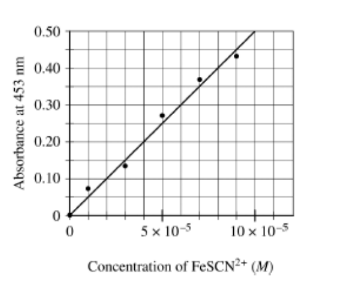
If the absorbance of the mixture is 0.20 at 453 nm, how many moles of \(Fe^{3+}\)(aq) were present in the 100. mL sample? (Assume that any volume change due to adding the KSCN(s) is negligible.)
(A) \(4×10^{-4}\) mol
(B) \(3×10^{-4}\) mol
(C) \(4× 10^{-6}\) mol
▶️Answer/Explanation
Ans:A
Question
Which of the following pieces of laboratory glassware should be used to most accurately measure out a 25.00 mL sample of a solution?
(A) 5 mL pipet
(B) 25 mL pipet
(C) 25 mL beaker
(D) 25 mL Erlenmeyer flask
(E) 50 mL graduated cylinder
▶️Answer/Explanation
Ans: B