Question

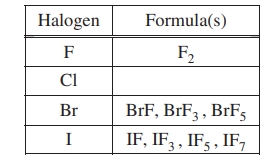
Some binary compounds that form between fluorine and various nonmetals are listed in the table above. A student examines the data in the table and poses the following hypothesis: the number of F atoms that will bond to a nonmetal is always equal to 8 minus the number of valence electrons in the nonmetal atom.
(a) Based on the student’s hypothesis, what should be the formula of the compound that forms between chlorine and fluorine?
(b) In an attempt to verify the hypothesis, the student researches the fluoride compounds of the other halogens and finds the formula \(CIF_{3}\). In the box below, draw a complete Lewis electron-dot diagram for a molecule of\( CIF_{3}\).

(c) Two possible geometric shapes for the\( CIF_{3}\) molecule are trigonal planar and T-shaped. The student does some research and learns that the molecule has a dipole moment. Which of the two shapes is consistent with the fact that the \( CIF_{3}\) molecule has a dipole moment? Justify your answer in terms of bond polarity and molecular structure.
In an attempt to resolve the existence of the \( CIF_{3}\) molecule with the hypothesis stated above, the student researches the compounds that form between halogens and fluorine, and assembles the following list.
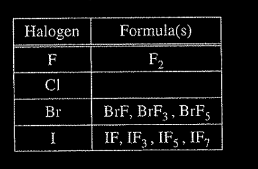
(d) Based on concepts of atomic structure and periodicity, propose a modification to the student’s previous hypothesis to account for the compounds that form between halogens and fluorine.
▶️Answer/Explanation
(a) \(CLF\)
(b) 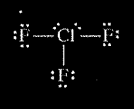
(c) The molecule is T-shaped because a T-shaped structure is asymmetric with dipoles that do not cancel out, but produce a net dipole (i.e., a polar molecule).
OR
because, if the molecule had a trigonal planar structure, the molecule would be symmetric with dipoles that cancel out and produce a net dipole of zero (i.e., a nonpolar molecule), which is not consistent with the observation that the CIF3 molecule does have a dipole moment.
(d) An acceptable hypothesis (descriptive or formulaic) must include the following ideas:
1. Atomic Structure; e.g., odd number of F atoms
2. Periodicity: e.g., as the atomic number of the central halogen atom increases, the number of F atoms increases.
Question
Answer the following questions relating to Fe and its ions, Fe2+ and Fe3+.
(a) Write the ground-state electron configuration of the Fe2+ ion.
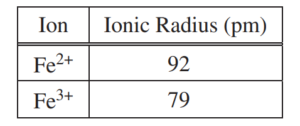
(b) The radii of the ions are given in the table above. Using principles of atomic structure, explain why the radius of the Fe2+ ion is larger than the radius of the Fe3+ ion.
(c) Fe3+ ions interact more strongly with water molecules in aqueous solution than Fe2+ ions do. Give one reason for this stronger interaction, and justify your answer using Coulomb’s law.
A student obtains a solution that contains an unknown concentration of Fe2+(aq). To determine the concentration
of Fe2+(aq) in the solution, the student titrates a sample of the solution with MnO4 –(aq) , which converts Fe2+(aq) to Fe3+(aq), as represented by the following equation.
5 Fe2+(aq) + MnO4−(aq) + 8 H+(aq) → 5 Fe3+(aq) + Mn2+(aq) + 4 H2O(l)
(d) Write the balanced equation for the half-reaction for the oxidation of Fe2+(aq) to Fe3+(aq).
(e) The student titrates a 10.0 mL sample of the Fe2+(aq) solution. Calculate the value of [Fe2+] in the solution if it takes 17.48 mL of added 0.0350 M KMnO4(aq) to reach the equivalence point of the titration.
To deliver the 10.0 mL sample of the Fe2+(aq) solution in part (e), the student has the choice of using one of the pieces of glassware listed below.
• 25 mL buret • 25 mL beaker
• 25 mL graduated cylinder • 25 mL volumetric flask
(f) Explain why the 25 mL volumetric flask would be a poor choice to use for delivering the required volume of the Fe2+(aq) solution.
In a separate experiment, the student is given a sample of powdered Fe(s) that contains an inert impurity. The student uses a procedure to oxidize the Fe(s) in the sample to Fe2O3(s). The student collects the following data during the experiment.
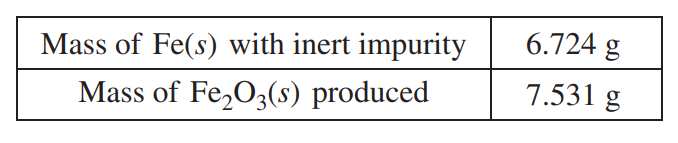
(g) Calculate the number of moles of Fe in the Fe2O3(s) produced.
(h) Calculate the percent by mass of Fe in the original sample of powdered Fe(s) with the inert impurity.
(i) If the oxidation of the Fe(s) in the original sample was incomplete so that some of the 7.531 g of product was FeO(s) instead of Fe2O3(s), would the calculated mass percent of Fe(s) in the original sample be higher, lower, or the same as the actual mass percent of Fe(s)? Justify your answer
▶️Answer/Explanation
Ans:
(a)
| 1s2 2s2 2p6 3s2 3p6 3d6 OR [Ar] 3d6 |
(b)
| Both ions have the same nuclear charge; however, the greater number of electrons in the outermost shell of Fe2+ results in greater electron – electron repulsion that shell, leading to a larger radius. |
(c)
| Coulomb’s law : \(F \propto \frac{q_{1}q_{2}}{r^{2}}\) (need not be explicitly stated) IN comparison to the Fe2+ ion, the Fe3+ ion has a higher charge. OR The smaller size of Fe3+ allows it to get closer to a water molecule. |
(d)
| Fe2+(aq) → Fe3+ (aq) + e– |
(e)
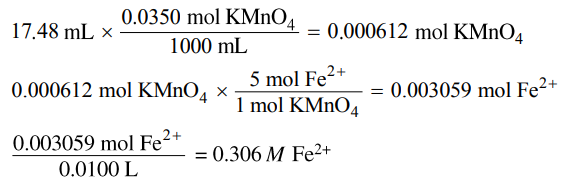 |
(f)
| The volumetric flask is designed to contain only 25.00 mL precisely. |
(g)
7.531 g Fe2O3 × \(\frac{1 mol Fe_{2}O_{3}}{159.70 g Fe_{2}O_{3}}\) = 0.04716 mol Fe2O3 0.04716 mol Fe2O3 × \(\frac{2 mol Fe}{1 mol Fe_{2}O_{3}}\) = 0.09431 mol Fe |
(h)
0.09431 mol Fe × \(\) = 5.267 g Fe \(\frac{5.267 g Fe}{6.724 g Sample}\times 100= 78.33%\) |
(i)
The calculated mass percent of Fe would be lower than the actual mass percent of Fe. A sample that contains any FeO (rather than Fe2O3) will have a higher actual mass percent of Fe than a completely oxidized sample would have. Therefore, when the moles of Fe are calculated (assuming all the mass of the sample is Fe2O3) the calculated number of moles of Fe, and hence the calculated mass percent of Fe, will be lower. |
Question
Answer the following questions about Mg(OH)2 . At 25°C, the value of the solubility product constant, Ksp, for Mg(OH)2(s) is 1.8 × 10−11 .
(a) Calculate the number of grams of Mg(OH)2 (molar mass 58.32 g/mol) that is dissolved in 100. mL of a saturated solution of Mg(OH)2 at 25°C.
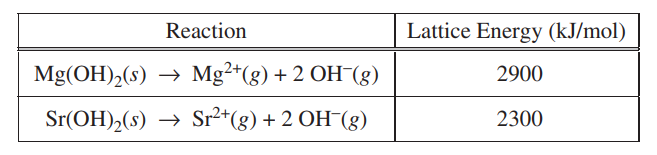
(b) The energy required to separate the ions in the Mg(OH)2 crystal lattice into individual Mg2+(g) and OH−(g) ions, as represented in the table below, is known as the lattice energy of Mg(OH)2(s). As shown in the table, the lattice energy of Sr(OH)2(s) is less than the lattice energy of Mg(OH)2(s). Explain why in terms of periodic properties and Coulomb’s law.
▶️Answer/Explanation
Ans:
(a)
1.8 × 10-11 = [Mg2+][OH–]2 = (x) (2x)2 = 4x3 \(x = \sqrt[3]{\frac{1.8\times 10^{-11}}{4}}=1.65\times 10^{-4}M=[Mg^{2+}]=[Mg(OH)_{2}]\) \(0.100 L\times \frac{1.65 \times 10^{-4}mol}{1L}\times \frac{58.32 g Mg(OH)_{2}}{1 mol Mg(OH)_{2}}=9.6\times 10^{-4} gMg(OH)_{2}\) |
(b)
| The Sr2+ ion is larger than the Mg2+ ion because it has additional occupied energy levels (or shells). Coulomb’s law states that the force of attraction between cation and anion is inversely proportional to the square of the distance between them. Since the distance between Mg2+ and OH– is shorter than the distance between Sr2+ and OH–, the attractive forces in Mg(OH)2 are stronger and, therefore, its lattice energy is greater. |
Question
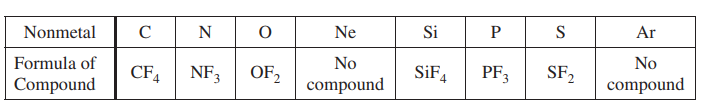
Some binary compounds that form between fluorine and various nonmetals are listed in the table above. A student examines the data in the table and poses the following hypothesis: the number of F atoms that will bond to a nonmetal is always equal to 8 minus the number of valence electrons in the nonmetal atom.
(a) Based on the student’s hypothesis, what should be the formula of the compound that forms between chlorine and fluorine?
(b) In an attempt to verify the hypothesis, the student researches the fluoride compounds of the other halogens and finds the formula ClF3 . In the box below, draw a complete Lewis electron-dot diagram for a molecule of ClF3 .
(c) Two possible geometric shapes for the ClF3 molecule are trigonal planar and T-shaped. The student does some research and learns that the molecule has a dipole moment. Which of the two shapes is consistent with the fact that the ClF3 molecule has a dipole moment? Justify your answer in terms of bond polarity and molecular structure.
In an attempt to resolve the existence of the ClF3 molecule with the hypothesis stated above, the student researches the compounds that form between halogens and fluorine, and assembles the following list.
(d) Based on concepts of atomic structure and periodicity, propose a modification to the student’s previous hypothesis to account for the compounds that form between halogens and fluorine.
▶️Answer/Explanation
Ans:
(a)
| CIF |
(b)
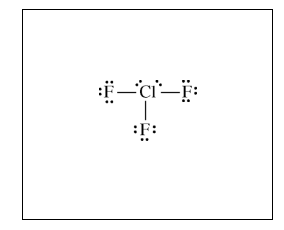
| See diagram above |
(c)
| The molecule is T-shaped because a T-shaped structure is asymmetric with dipoles that do not cancel out, but produce a net dipole (i.e., a polar molecule). OR because, if the molecule had a trigonal planar structure, the molecule would be symmetric with dipoles that cancel out and produce a net dipole of zero (i.e., a nonpolar molecule), which is not consistent with the observation that the ClF3 molecule does have a dipole moment. |
(d)
| An acceptable hypothesis (descriptive or formulaic) must include the following ideas: 1. Atomic Structure: e.g., odd number of F atoms 2. Periodicity: e.g., as the atomic number of the central halogen atom increases, the number of F atoms increases. |
