Question
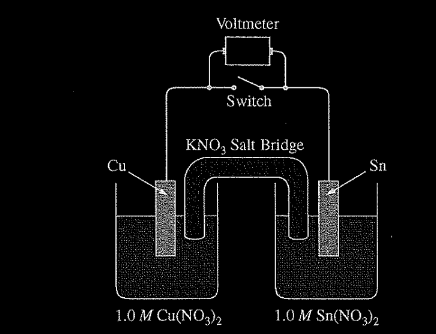
A student is given a standard galvanic cell, represented above, that has a Cu electrode and a Sn electrode. As current flows through the cell, the student determines that the Cu electrode increases in mass and the Sn electrode decreases in mass.
(a) Identify the electrode at which oxidation is occurring. Explain your reasoning based on the student’s observations.
(b) As the mass of the Sn electrode decreases, where does the mass go?
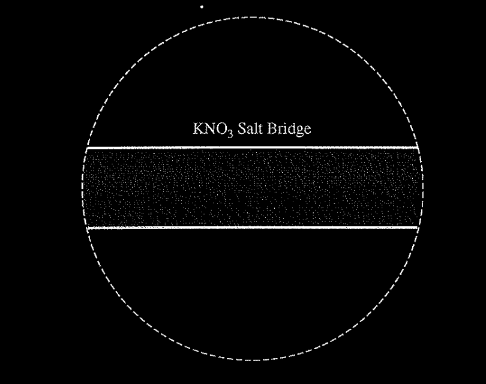
(c) In the expanded view of the center portion of the salt bridge shown in the diagram below, draw and label a particle view of what occurs in the salt bridge as the cell begins to operate. Omit solvent molecules and use arrows to show the movement of particles.
d) A nonstandard cell is made by replacing the 1.0 M solutions of Cu\((NO_3)_2\) and Sn\((NO_3)_2\) in the standard cell with 0.50 M solutions of Cu(NO3)2 and Sn\((NO_{3})_{2}\). The volumes of solutions in the nonstandard cell are identical to those in the standard cell.
(i) Is the cell potential of the nonstandard cell greater than, less than, or equal to the cell potential of the standard cell? Justify your answer.
(ii) Both the standard and nonstandard cells can be used to power an electronic device. Would the nonstandard cell power the device for the same time, a longer time, or a shorter time as compared with the standard cell? Justify your answer.
(e) In another experiment, the student places a new Sn electrode into a fresh solution of 1.0 M\( Cu(NO_{3})_{2}\) ·
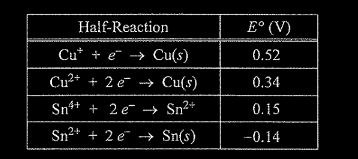
(i) Using information from the table above, write a net-ionic equation for the reaction between the Sn electrode and the Cu\((NO_3)_2\) solution that would be thermodynamically favorable. Justify that the reaction is thermodynamically favorable.
(ii) Calculate the value of AG for the reaction. Include units with your answer.
▶️Answer/Explanation
(a) Since the Sn electrode is losing mass, Sn atoms must be forming \(Sn^{2+}\)(aq). This process is oxidation. OR because the cell operates, E° must be positive and, based on the E° values from the table, it must be Sn that is oxidized.
(b) The atoms on the Sn electrode are going into the solution as \(Sn^{2+}\) ions.
(c) The response should show at least one \(K^+\) ion moving toward the Cu compartment on the left and at least one \(NO_{3}^-\) ion moving in the opposite direction.
d(i) It is the same. In the cell reaction \(Q= \frac{[Sn^{2+}]}{ [Cu^{2+}]}\) , and the concentrations of \(Sn^{2+}\) and \(Cu^{2+}\) are equal to each other in both cases.
d(ii) The nonstandard cell would power the device for a shorter time because the supply of \(Cu^{2+}\) ions will be exhausted more quickly. OR The nonstandard cell would power the device for a shorter time because the reaction will reach E=0 more quickly.
e (i) \(Cu^{2+}(aq) + Sn(s) → Cu(s) + Sn^{2+}\)(aq) E° is positive \((0.34 V + 0.14 V = 0.48 V)\), therefore the reaction is thermodynamically favorable. OR The cell observations from earlier parts of the question are evidence that the Sn is oxidized and Cu is reduced, therefore E° must be positive.
e.(ii) \(\Delta G^0=-nFE^0\)
\(\Delta G^0=-\frac{2 mol e^-}{mol_{rxn}}\times \frac{96,485}{mol e^-}\times \frac{0.48}{c}=-93,000 J/mol_{rxn}\)
Question.
\(2NaHCO_{3}(s)\rightarrow Na_{2}CO_{3}+CO_{2}(g)+H_{2}O(g)\)
\( NaHCO_3\)(s) (baking soda) decomposes upon heating to produce \(Na_2CO_3(s) \)and two gaseous products, as shown by the equation above.
(a) A student claims that the reaction is an oxidation-reduction reaction because the oxidation number of carbon changes. Do you agree with the claim? In your answer include the oxidation number of carbon in each of the three carbon-containing species in the reaction. The student conducts an experiment to determine the composition of a mixture of \(NaHCO_3\) (molar mass 84.01 g/mol) and \(Na_2CO_3\) (molar mass 105.99 g/mol). The student places a sample of the mixture into a preweighed test tube that is attached to a container that holds a drying agent. The student heats the test tube strongly with a Bunsen burner for 10 minutes, during which time all of the water produced by the reaction is captured by the drying agent. The following table shows the data the student recorded during the experiment.

(b) Calculate the number of moles of\( NaHCO_3(s)\) present in the mixture in the test tube before the reaction was initiated.
(c) Determine the mass percent of\( NaHCO_3(s)\) in the mixture.
(d) If the student spills some of the mixture out of the test tube after weighing the test tube and mixture and before heating, how would this error affect the mass percent of \(NaHCO_3\) calculated in part (c) ? Justify your answer. When a sample of pure\( Na_2CO_3 \)is placed in distilled water, the student observes that the pH of the solution increases significantly. This process is represented by the balanced net-ionic equation shown below.
\(CO_{3}^{2-}(aq)+H_2O{l}\rightleftharpoons HCO_{3}^-+OH^{-}(aq)\)
(e) The student prepares a 0.10 M \(Na_2CO_3(aq)\) solution and measures the pH of the solution to be 11.65.
(i) Calculate\( [OH^{-}]\) in the \(Na_2CO_3\)(aq) solution.
(ii) Write the expression for \(K_b\) for the carbonate ion.
(iii) Calculate the value of \(K_b \)for the carbonate ion. The student adds some 1.0 M Sr\((NO_3)_2\)(aq) to the 0.10 M\( Na_2CO_3(aq)\) and observes the formation of a precipitate.
(f) Write the balanced net-ionic equation for the reaction between Sr\((NO_3)_2\) and \(Na_2CO_3\) that produces the precipitate.
▶️Answer/Explanation
(a) No. The oxidation number of carbon is +4 in each of the three carbon-containing species.
(b)
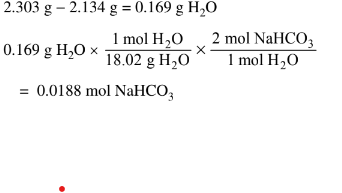
(c) \(0.0188mol NaHCO_{3}\times \frac{84.01g NaHCO_{3}}{1molNaHCO_{3}}\frac{1.58g}{(17.648g-15.825g)}\times =86.7%\)
(d) The calculated mass percent would be smaller than the actual value. Less water would be collected, so there would appear to be less\( NaHCO_3\) in the sample.
(e)(i) \(pOH = 14.00 – 11.65 = 2.35\)
\([OH^−] = 10-2.35 = 4.5\times 10^-{3}\)
e(ii) \(k_b=\frac{[HCO_{3}^{-}][OH^-]}{CO_{3}^{2-}}\)
e(iii) \(K_b=\frac{(4.5\times 10^{-3})\times (4.5\times 10^{-3})}{0.10-(4.5\times 10^{-3})}\approx \frac{(4.5\times 10^{-3})^2}{0.10}= 2.0\times 10^{-4}\)
(f) \(Sr^{2+}CO_{3}^{2-}\rightarrow SrCO_{3}\)
Question
Answer the following questions relating to HCl,\(CH_3Cl,\) and \(CH_3\)Br.
(a) HCl(g) can be prepared by the reaction of concentrated \(H_ 2SO_4\)(aq) with NaCl(s), as represented by the following equation.
\(H_{2}SO_{4}(aq)+2NaCl(s)\rightarrow 2HCl(g)+Na_{2}SO_{4}(aq)\)
(i) A student claims that the reaction is a redox reaction. Is the student correct? Justify your answer.
(ii) Calculate the mass, in grams, of NaCl(s) needed to react with excess \(H_2SO_4\)(aq) to produce 3.00 g of HCl(g). Assume that the reaction goes to completion.HCl(g) can react with methanol vapor,\( CH_3OH(g)\), to produce \(CH_3Cl\)(g), as represented by the following equation.
\(CH_{3}OH(g)+HCl(g)\rightleftharpoons CH_{3}Cl(g)+H_{2}O(g) K_{P}=4.7\times 10^{3}\) At400K
(b) \(CH _3OH(g)\) and HCl(g) are combined in a 10.00 L sealed reaction vessel and allowed to reach equilibrium at 400 K. The initial partial pressure of\( CH_3OH(g)\) in the vessel is 0.250 atm and that of HCl(g) is 0.600 atm.
(i) Does the total pressure in the vessel increase, decrease, or remain the same as equilibrium is approached? Justify your answer in terms of the reaction stoichiometry.
(ii) Considering the value of Kp , calculate the final partial pressure of HCl(g) after the system inside the vessel reaches equilibrium at 400 K.
(iii) The student claims that the final partial pressure of \(CH_3OH(g) \)at equilibrium is very small but not exactly zero. Do you agree or disagree with the student’s claim? Justify your answer.
(c) The table below shows some data for the compounds \(CH _3Cl \)and \(CH_3Br\).
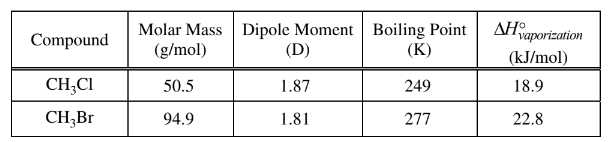
(i) Identify all the types of intermolecular forces that exist among molecules in \(CH_3Cl(l)\).
(ii) In terms of intermolecular forces, explain why the boiling point of CH3Br(l) is greater than that of \(CH_3Cl(l)\).
(d) A 2.00 mL sealed glass vial containing a 1.00 g sample of \(CH_3Cl(l)\) is stored in a freezer at 233 K.
(i) Calculate the pressure in the vial at 298 K assuming that all the \(CH_3Cl(l)\)vaporizes.
(ii) Explain why it would be unsafe to remove the vial from the freezer and leave it on a lab bench at 298 K.
▶️Answer/Explanation
a(i) No, the student is not correct. None of the oxidation numbers of the elements change (H = +1, S = +6, O = -2, Na = +1, Cl = -1).
a(ii) 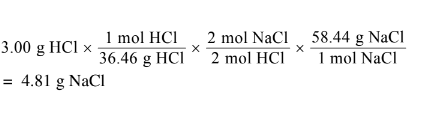
(b)(i) The pressure will remain the same. The reaction stoichiometry shows that two moles of gaseous reactants produce two moles of gaseous products. Because the number of moles of gas does not change, the pressure does not change.
b(ii)The value of\( K_p\) is large, so the reaction will proceed to the right until the limiting reactant is essentially used up. Thus practically all of the \(CH_3OH(g)\) will react and the final pressure of HCl(g) is 0.600 – 0.250 = 0.350 atm.
OR
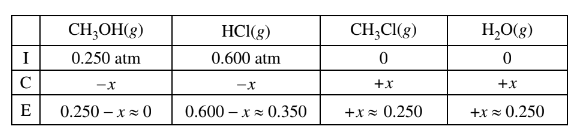
The final pressure of HCl(g) is 0.350 atm at equilibrium.
b(iii) Agree. The large value of \(K_p\) means that the partial pressure of the limiting reactant at equilibrium will be extremely small, but some \(CH_3OH \)molecules must exist for the system to be in dynamic equilibrium.
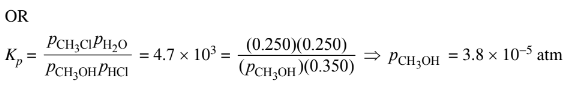
The partial pressure of \(CH_3OH(g)\) is very small but is not zero.
c(i) London dispersion forces and dipole-dipole forces
c(ii) The electron cloud in \(CH_3Br\) is larger and more polarizable than that of \(CH_3Cl\). As a result the London dispersion forces are stronger in \(CH_3Br\) compared to those in \(CH_3Cl\)and consequently the boiling point of\( CH_3\)Br is higher than that of \(CH_3Cl\).
d(i)
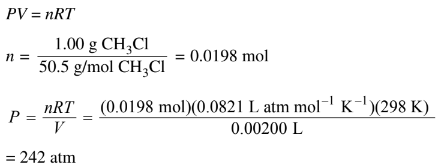
d(ii) At room temperature the liquid will vaporize. Consequently the glass vial may not be strong enough to withstand the increase in pressure.
