Question
Answer the following questions about the element Si and some of its compounds.
(a) The mass spectrum of a pure sample of Si is shown below.
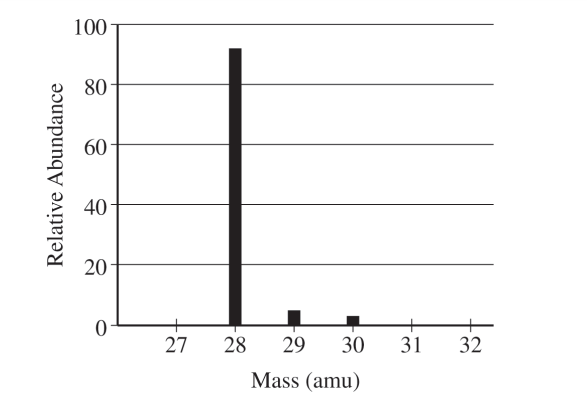
(i) How many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of Si ?
(ii) Write the ground-state electron configuration of Si.
Two compounds that contain Si are SiO2 and SiH4.
(b) At 161 K, SiH4 boils but SiO2 remains as a solid. Using principles of interparticle forces, explain the difference in boiling points.
At high temperatures, SiH4 decomposes to form solid silicon and hydrogen gas.
(c) Write a balanced equation for the reaction.
A table of absolute entropies of some substances is given below.
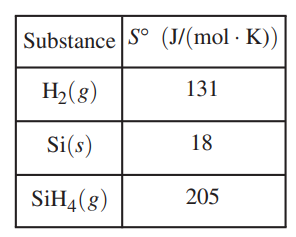
(d) Explain why the absolute molar entropy of Si(s) is less than that of H2(g).
(e) Calculate the value, in J/(mol. K), of ∆S° for the reaction.
(f) The reaction is thermodynamically favorable at all temperatures. Explain why the reaction occurs only at high temperatures.
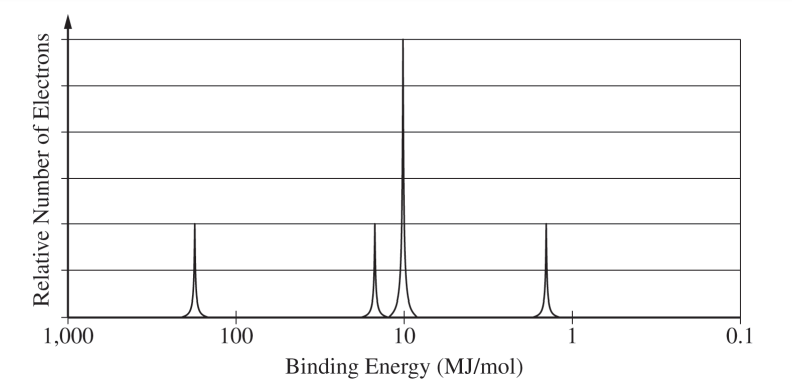
(g) A partial photoelectron spectrum of pure Si is shown below. On the spectrum, draw the missing peak that corresponds to the electrons in the 3p sublevel.
(h) Using principles of atomic structure, explain why the first ionization energy of Ge is lower than that of Si.
(i) A single photon with a wavelength of 4.00 × 10−7 m is absorbed by the Si sample. Calculate the energy of the photon in joules.
▶️Answer/Explanation
Ans:
(a) (i) For the correct answer:
14 protons and 14 neutrons
(ii) For the correct answer:
Accept one of the following:
• 1s2 2s2 2p6 3s2 3p2
• [Ne] 3s2 3p2
(b) For a correct explanation:
SiH4 is composed of molecules, for which the only intermolecular forces are London dispersion forces. SiO2 is a network covalent compound with covalent bonds between
silicon and oxygen atoms. London dispersion forces are much weaker than covalent bonds, so SiH4 boils at a much lower temperature than SiO2.
(c) For the correct balanced equation (state symbols not required):
SiH4 ( g) → Si(s) + 2 H2(g)
(d) For a correct explanation:
The H2(g) molecules are more highly dispersed than the Si(s) atoms and, therefore, have a higher absolute molar entropy. Silicon is a solid; therefore, its atoms are in fixed positions,
are less dispersed, and have a lower absolute molar entropy.
(e) For the correct calculated value:
ΔS0rxn = (18 + 2(131)) – 205 = +75 J/(molrxn • K)
(f) For a correct explanation:
High temperature is required for the reactant particles to have sufficient thermal energy to overcome the activation energy of the reaction.
(g) For the correct peak height and location:
The peak should be drawn to the right of the other peaks, and it should reach the second line above the horizontal axis.
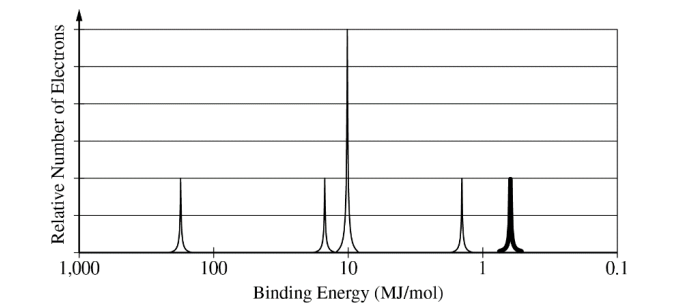
(h) For a correct explanation:
The valence electrons of a Ge atom occupy a higher shell (n=4) than those of a Si atom (n=3), so the average distance between the nucleus and the valence electrons is greater in
Ge than in Si. This greater separation results in weaker Coulombic attractions between the Ge nucleus and its valence electrons, making them less tightly bound and, therefore, easier
to remove compared to those in Si.
(i) For the correct calculated value:
\(E = hv = h\left ( \frac{c}{\lambda } \right )=6.626\times 10^{-34}J\cdot s\left ( \frac{2.998\times 10^{8}ms^{-1}}{4.00\times 10^{-7}m} \right )=4.97 \times 10^{-19} J\)
