Question
\(3Ag(s)+4HNO_{3}(aq)\rightarrow 3AgNO_{3}(aq)+NO(g)+2H_{2}O(l)\)
A student investigates the reaction between Ag(s) and \(HNO_3\)(aq) represented by the equation above.
(a) Predict the sign of the entropy change, ΔS°, for the reaction. Justify your answer.
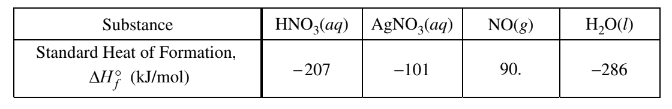
(b) Use the information in the table below to calculate the value of \(\Delta H^{\circ}_{rxn}\), the standard enthalpy change for the reaction, in\( kJ/mol_{rxn}\) .
(c) Based on your answers to parts (a) and (b), is the reaction more likely to be thermodynamically favorable at 25°C, or at 95°C? Justify your answer.
(d) The student runs the reaction using a 3 to 4 mole ratio of Ag(s) to\( HNO_3\)(aq). Suggest a method the student can use to isolate solid \(AgNO_3 \)from the other products of the reaction.
▶️Answer/Explanation
(a) The entropy change is positive because the reaction has one mole of gas in the products and none in the reactants.
(b)\( \Delta H^{\circ}_{rxn} = 3(-101) + 90. +2(-286) -4(-207)
= 43 kJ/molrxn\)
(c) \(\Delta G^{\circ}=\Delta H^{\circ}-T\Delta S^{\circ}\) The reaction is more likely to be favorable at \(95G^{\circ}\). At the higher temperature, the term\( T\Delta S^{\circ}\) is larger and positive; thus, when subtracted from \(\Delta H^{\circ}\), the value of \(\Delta G^{\circ} \) is more likely to be negative.
(d) The student can evaporate the water, leaving behind solid silver nitrate.
Question
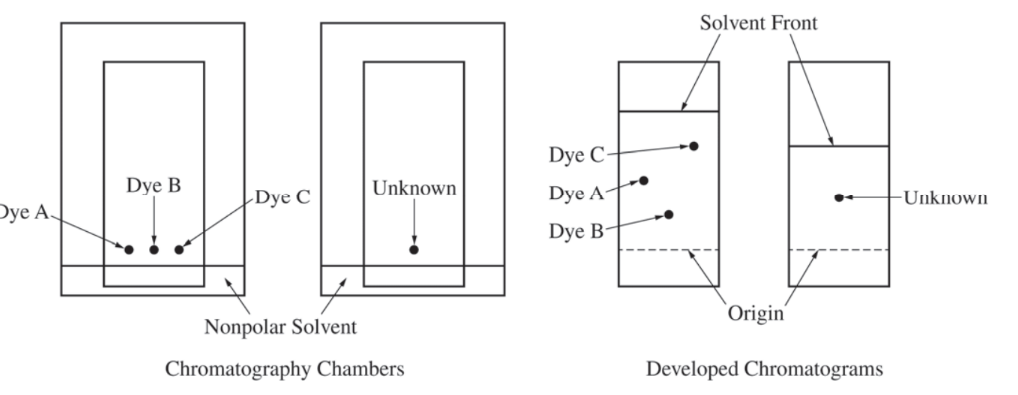
A student investigates various dyes using paper chromatography. The student has samples of three pure dyes, labeled A, B, and C, and an unknown sample that contains one of the three dyes. The student prepares the chromatography chambers shown above on the left by putting a drop of each dye at the indicated position on the chromatography paper (a polar material) and standing the paper in a nonpolar solvent. The developed chromatograms are shown above on the right.
(a) Which dye (A, B, or C) is the least polar? Justify your answer in terms of the interactions between the dyes and the solvent or between the dyes and the paper.
(b) Which dye is present in the unknown sample? Justify your answer.
▶️Answer/Explanation
Ans:
(a)
Dye C is the least polar because it moved the farthest. Nonpolar dyes are more strongly attracted to the nonpolar solvent. |
(b)
Dye A is present in the unknown sample. The unknown sample moves to a position that is midway between the origin and the solvent front, and so does dye A. |
