Question
Nitrogen dioxide, \(NO_2~(g)\), is produced as a by-product of the combustion of fossil fuels in internal combustion engines. At elevated temperatures NO2(g) decomposes according to the equation below.
\(2 NO_2(g) → 2 NO~(g) + O_2~(g)\)
The concentration of a sample of \(NO_2~(g)\) is monitored as it decomposes and is recorded on the graph directly below. The two graphs that follow it are derived from the original data
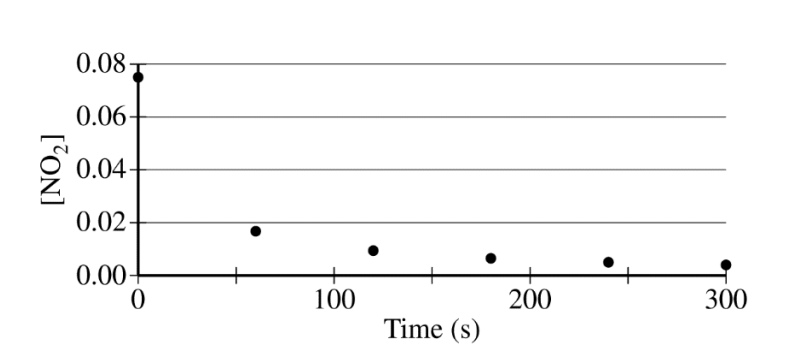
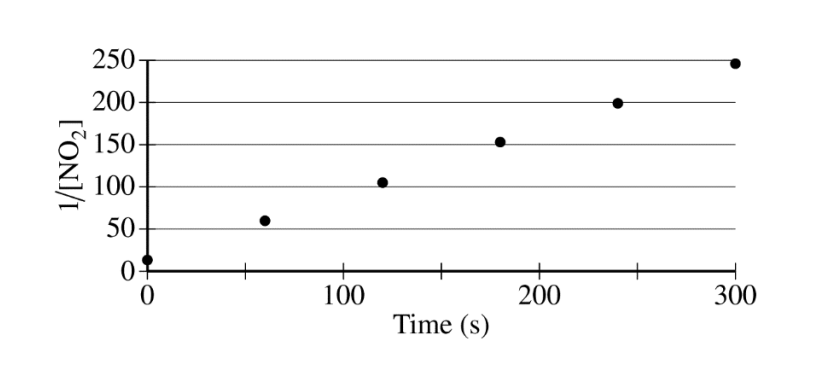
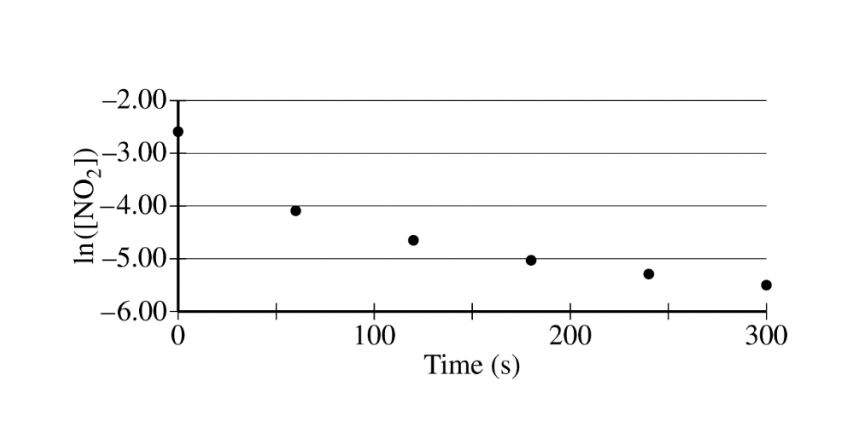
(a) Explain how the graphs indicate that the reaction is second order.
(b) Write the rate law for the decomposition of NO2(g).
(c) Consider two possible mechanisms for the decomposition reaction.
(i) Is the rate law described by mechanism I shown below consistent with the rate law you wrote in part (b) ? Justify your answer.
Mechanism I
Step 1: NO2(g) + NO2(g) → NO(g) + NO3(g) slow
Step 2: NO3(g) → NO(g) + O2(g) fast
(ii) Is the rate law described by mechanism II shown below consistent with the rate law you wrote in part (b) ? Justify your answer.
Mechanism II
Step 1: NO2(g) + NO2(g) ⇔ N2O4(g) fast equilibrium
Step 2: N2O4(g) → 2 NO(g) + O2(g) slow
▶️Answer/Explanation
Ans:
(a)
| The linear graph of \(\frac{1}{[NO_{2}]}\) vs. time indicates a second-order reaction. |
(b)
| rate = k[NO2]2 |
(c) (i)
| Yes, Step 1 is slow, therefore it is the rate-determining step of this mechanism. The rate law of this elementary reaction is rate = k[NO2][NO2] = k[NO2]2, which is consistent with the second – order rate law in part (b). |
(ii)
| Yes, Step 2 is slow; therefore, it is the rate – determining step of this mechanism. The rate law of this elementary reaction is rate = k[N2O2]. Because N2O4 is an intermediate, it cannot appear in the rate law of the overall reaction. Because \(K_{eq}\frac{[N_{2}O_{4}]}{[NO_{2}]^{2}}\) in step 1, [N2O4] = Keq[NO2]2. Then, substituting Keq[NO2]2 for [N2O4] in the rate law of step 2 gives rate = (k Keq) [NO2]2 , which is consistent with the rate law in part (b). |
